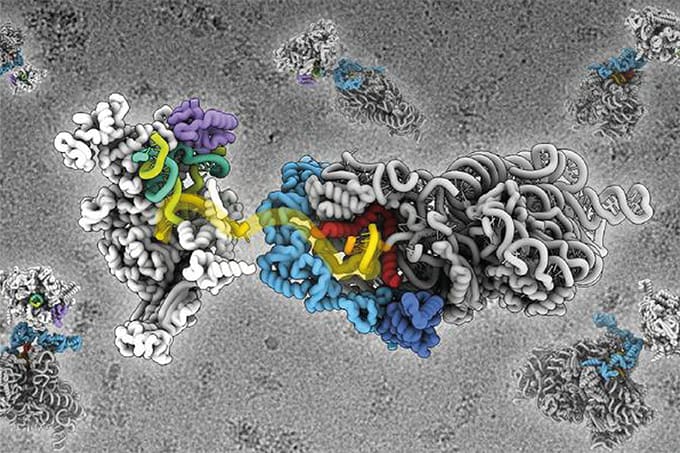
Researchers at MIT have developed a hybrid microscopy system that can image neuronal and metabolic activity with single-cell precision at unprecedented depths in brain tissue. The method surpasses the limits of traditional fluorescence microscopy by using a blend of three-photon excitation and ultrasound detection.
The system was able to visualize NAD(P)H – a key metabolic molecule linked to neuronal activity – through samples up to 1.1 mm thick, including mouse brain slices and human-derived cerebral organoids. That’s more than five times deeper than existing optical imaging methods can reliably detect NAD(P)H in dense brain tissue. The researchers believe they could have gone even deeper, but the test samples were simply not thick enough. “I think we’re pretty confident about going deeper,” said co-lead author W. David Lee in a press release.
Key to the advance is a three-part innovation the team dubs “Multiphoton-In and Acoustic-Out.” Instead of using conventional fluorescent labels, the system excites target molecules with tightly focused, ultrashort pulses of light at triple their absorption wavelength – a three-photon process that minimizes tissue scattering and penetrates deeper. Although this produces only a weak fluorescent signal, it generates localized thermal expansion inside the cell. That expansion emits sound waves, which are picked up by a sensitive ultrasound microphone and converted into images.
This combination of three-photon excitation, label-free detection, and photoacoustic imaging enabled the team to track cell metabolism without dyes or genetic modifications. The system also supported third-harmonic generation imaging – capturing structural details of cells simultaneously with their metabolic signals.
Because the new method doesn’t require chemical or genetic labels, it could eventually be used in human subjects – for instance, during neurosurgery. Metabolic indicators like NAD(P)H are altered in Alzheimer’s disease, Rett syndrome, and seizures, making them promising biomarkers for neurological monitoring. “The major advance here is to enable us to image deeper at single-cell resolution,” said Mriganka Sur, a co-corresponding author at MIT’s Picower Institute.
The next milestone will be in vivo validation, as current experiments were done in vitro or on ex vivo tissue. The challenge is integrating the acoustic detector on the same side as the light source – necessary for living systems. But based on current results, the team believes 2 mm imaging depth in live brain tissue is within reach.