According to ICH guideline Q3A (R2), impurities in new drug substances at levels of 0.05 % or above must be reported, and impurities at 0.1 % or above must be identified. Enantiomers of chiral drugs often show differences in pharmacokinetic behavior and pharmacological activity. One enantiomer might be pharmacologically active, while the other might be inactive, or even toxic. Therefore, the FDA has released guidance on the development of new stereo-isomeric drugs, demanding that the stereo-isomeric composition of a drug with a chiral center is known, and that specifications for the final product include assurance of purity from a stereochemical viewpoint.

The analysis of impurities contained in pharmaceutical substances can be accomplished by subjecting a concentrated solution of the substance to liquid chromatographic analysis. Impurities separated from the pharmaceutical substance are detected as small peaks next to a large peak originating from the main compound.
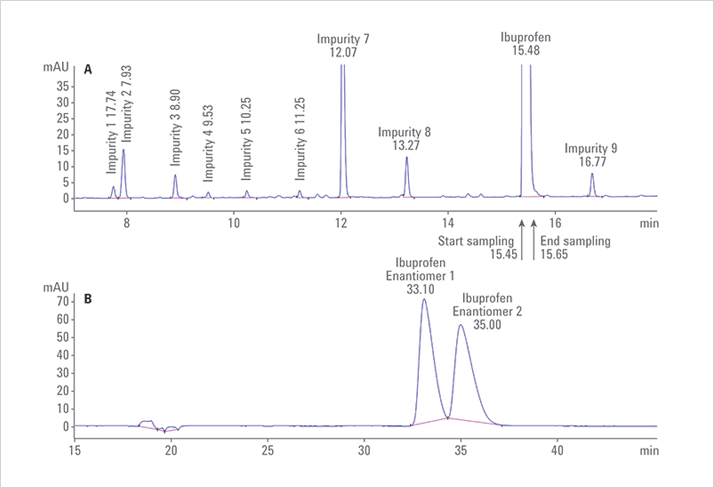
Racemic ibuprofen was chosen to prove the principle of the analysis of impurities in chiral pharmaceutical substances with simultaneous determination of the enantiomeric composition of the API. Figure 1A shows the chromatogram resulting from the first dimension reversed phase analysis of ibuprofen. Here, several impurities are separated from the
main compound.
The effluent from the first-dimension column was sampled at 15.45 minutes with a loop fill time of 0.20 minutes to transfer the ibuprofen peak to the second-dimension chiral column and enable separation of the enantiomers. Figure 1 shows when the effluent of the first-dimension column was cut and transferred to the second-dimension column (A) and the separation of the ibuprofen enantiomers on the second-dimension chiral column (B). The ibuprofen enantiomers were separated with a resolution of Rs = 1.25 on the second-dimension chiral column.
This article demonstrates that the Agilent 1290 Infinity 2D-LC Solution is ideally suited for the analysis of impurities in chiral pharmaceutical substances and for the simultaneous determination of the enantiomeric composition of the API. In the first dimension, a reversed phase separation was used to separate achiral impurities from the API. A heart-cutting experiment was used to transfer the API to a second-dimension chiral column for determination of the enantiomeric composition.
Agilent Technologies Inc.
5301 Stevens Creek Blvd., Santa Clara, California 95051, USA