During my undergraduate degree in organic chemistry at The University of Manchester Institute of Science and Technology, I was intrigued by my professor’s research into instrumentation and its application to the analysis of chemical compounds. I went on to choose a PhD focused on the design of mass spectrometers and later worked with Professor Dudley Williams at Cambridge University on the characterization of N-terminally blocked peptides by mass spectrometry, which were not amenable to standard Edman sequencing used at the time. At that time, LC-MS was not yet a routine technique, so it was a significant challenge to apply it to biological molecules, which include many polar or labile compounds. It was a very exciting time for the field of mass spectrometry!
Later, I was hired by Burrough Wellcome Co. in North Carolina, where LC-MS was being applied to the analysis of drugs in various phases of drug R&D. After many years in the pharmaceutical industry, I eventually found my way back to the analytical instrument side of the field, and have been with Agilent for the past 10 years.

Focusing on the life science and pharmaceutical arena, the biggest advance has been the ability to do routine analysis of biologically relevant compounds, such as drugs and metabolites. Typically polar and labile, these molecules are not amenable to direct analysis with GC-MS techniques, so there was a big gap in the market for LC-MS. The introduction of techniques such as electrospray ionization in the 1980s and 1990s, made LC-MS much more reliable and robust, and its use for routine analyses has grown steadily ever since. We’ve seen that reflected in the growth of conferences like ASMS, which has gone from under 300 attendees to over 6,000 – many of whom work in the biological sciences.
The continuing evolution of software systems able to deal with high-speed data acquisition and processing has also played a crucial role in promoting adoption of MS detection.
Mass spectrometry is widely applied in R&D – used by synthetic or medicinal chemists looking for a quick verification of compound synthesis. Drug metabolism groups use it to help understand the nature and extent of metabolic products, and it’s the gold standard analytical technique for DMPK and bioanalysis studies.
However, it is less commonly used in method development, where companies tend to adopt the “fit-for-purpose” approach of using tried and tested instrumentation rather than technology that may have more analytical capability than is required; for example, a routine method for screening a target drug once it has moved into development.
In pharma QC, UV-Vis detection is predominant. By the time drugs make their way through to this stage, the analytical characterization of the drug is very well understood, so companies want to apply the simplest, most robust methodology that meets their needs. For many purposes, UV-Vis may well be sufficient. In cases where unexpected peaks arise in the LC-UV chromatogram more analytical information is required to identify these compounds. Mass spectrometry can help identification in such cases. However, as MS technology evolves and becomes much more automated, I think analysts will gain confidence and start to apply it in more areas where greater specificity and sensitivity is required to identify eluting LC components.
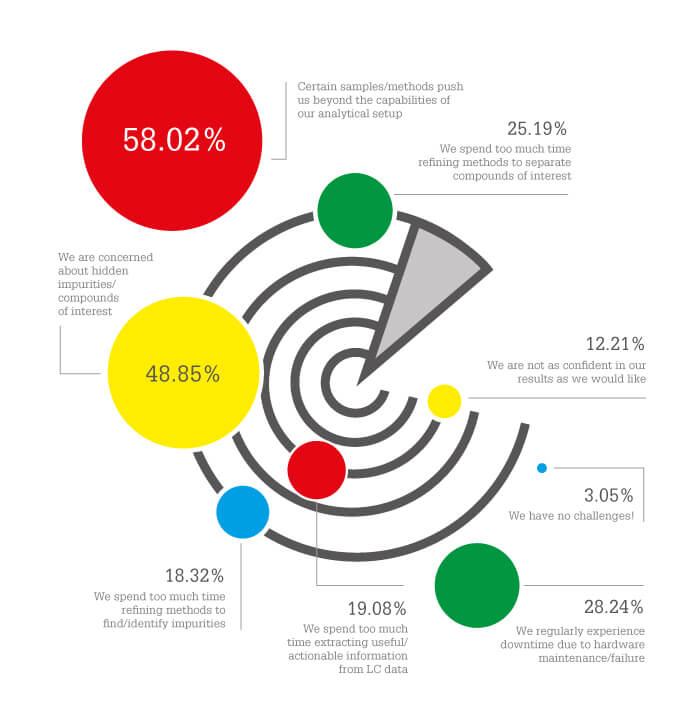
The Analytical Scientist reader survey represents a useful insight into the barriers and pain points that limit the use of MS.
Certain samples/methods push us beyond the capabilities of our analytical setup (58 percent)
Lester says: Some samples are much easier to deal with than others; for example, looking at drugs in matrix may be particularly challenging.
We are concerned about hidden impurities/compounds of interest (49 percent)
Lester says: Because of the exquisite sensitivity and selectivity of mass spectrometry as a detector, you can find very low levels of compounds that you may not have expected.
We regularly experience downtime due to hardware maintenance/failure (28 percent)
Lester says: Instrument downtime is always a huge area of frustration because (unlike the other two challenges) it is often outside your control...
Lester says: One reason for that, of course, is because users often have to go to either another lab or another instrument to use MS. If you had an MS detector on the same system as the UV-Vis detector then you would be able to utilize that information without having to switch instruments. The other factor is the cost – MS detection has traditionally been more expensive than UV-Vis. However, costs have come down dramatically over the past few years and I think we will see MS being used more as a first-line detector.
1. Limited budget – 52 percent
2. Insufficient time to train staff on new detection system/software – 18 percent
3. MS would be too complicated for analysts – 16 percent
4. Management don’t see the value – 11 percent
Lester says: I believe MS will be of significant value for many analyses currently carried out with UV-Vis, but there may be an opinion among non-mass spectrometrists that MS is not routine or is challenging for non-experts to use. To overcome these barriers, I think we need to focus on ease of use. Software is key, as we’ve seen with our Open Access LC/MS, which only requires use of a simple sample entry process.