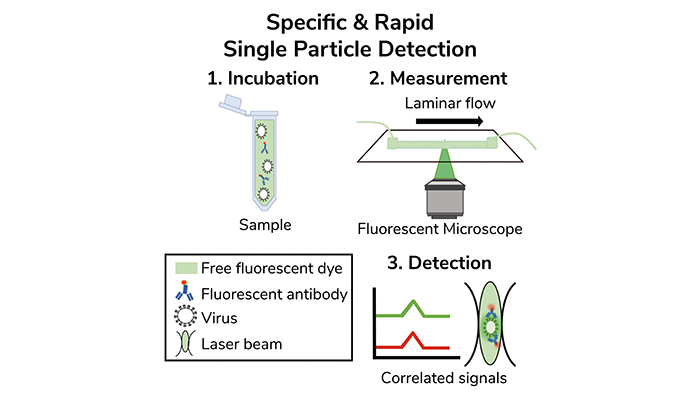
Confocal- & laminar flow-based detection scheme of intact virus particles, one at a time. Credit: Paz Drori
Microfluidic laminar flow combined with 3D-printed confocal fluorescence microscopy allows for the rapid and specific detection of individual virus particles and nanoparticles, according to new research. The method combines the precise control of fluid dynamics with high-resolution imaging to identify virus particles within minutes, offering an alternative to traditional virus detection techniques that can be slower and less sensitive.
The technique involves guiding samples through a microfluidic channel, where laminar flow enables controlled movement of particles through the confocal probe volume of the microscope. This setup allows the detection of both the physical size of the particles and their molecular signatures. "We developed a virus detection assay that functions as a sensitive and specific small-scale particle counter, relying on confocal-based detection in combination with microfluidic laminar flow," the researchers explain.
Current virus detection methods, such as polymerase chain reaction (PCR) and antigen-based tests, have limitations in sensitivity or require lengthy processing times. The flow-based virometer presented in this study can detect viruses one at a time, allowing for high specificity in virus identification based on their antigenic properties. This is achieved by correlating fluorescence signals from antibodies labeled with distinct fluorophores, which bind to viral epitopes, and the fluorescence signals from free dyes in the solution that indicate the volume of the particles.
"Using this approach, we were able to specifically detect SARS-CoV-2 and a recombinant vesicular stomatitis virus (rVSV-DG-spike), engineered to express the SARS-CoV-2 spike protein, within minutes," the authors noted. Detection times were minimized using a 3D-printed portable confocal setup, making this approach suitable for rapid screening of viruses, even in resource-limited environments.
To improve the sensitivity of the assay for low-concentration virus samples, the researchers introduced hydrodynamic focusing. This technique narrows the flow of the sample within the microfluidic channel, increasing the number of particles that pass through the detection zone, and enhancing the ability to detect viruses even at low particle concentrations. The team found that by using hydrodynamic focusing, they could achieve a sensitivity threshold relevant to clinical virus loads, such as those found in bodily fluids like saliva.
The study highlights the potential for this technology to be adapted for the detection of other bio-nanoparticles, such as exosomes or biomarkers related to cancer and neurodegenerative diseases. "The specific and sensitive counting of bio-NPs one-at-a-time, within minutes, using a simple assay and an affordable experimental setup could be considered for assisting in efficient diagnostics alongside existing traditional techniques," the study concludes.




