
Chromatography has undoubtedly reached a level of maturity, offering excellent performance for routine separations – and that’s why it has been widely adopted across various industries, such as pharmaceuticals, chemicals, environmental monitoring, and the food sector. However, there remains room for improvement in separating highly complex mixtures.
One fundamental challenge associated with chromatography stems from the intrinsic behavior of liquid flow within channels, where the liquid does not flow at a uniform velocity along the channel width. I’ve been thinking about this problem for quite some time – over a decade at this point. I spent my PhD and post-doc fellowship working on pillar array columns – attempting to reduce the pillar dimensions. Eventually, I realized that, when downscaling, defects and imperfections (even at the nanoscale level) put a limit on decreasing structure sizes much further.
So, I started thinking about some more exotic ways to enhance mass transport, so that large channels would behave as small channels, suffering less from the aforementioned fabrication and pressure drop limitations. After an extensive study of possible ways of achieving lateral mixing (including magnetic, acoustic, and electroosmotic principles) I landed on a potential solution: vortex chromatography. With some first considerations regarding feasibility, I submitted a Starting Grant at the European Research Council, which was accepted in 2015. This was the start of my exciting vortex chromatography journey.
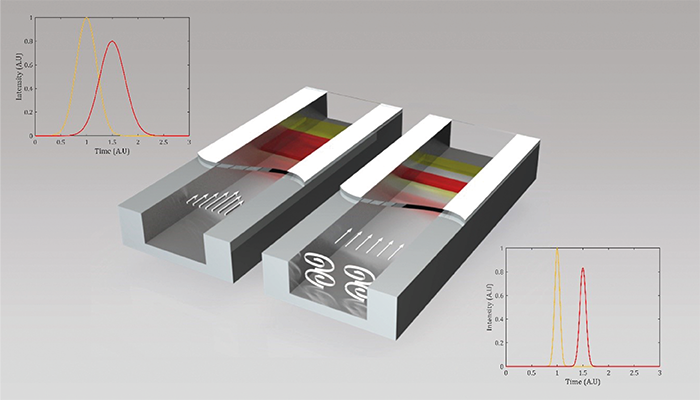
The solution developed by myself and colleagues at the µFlow group of the Vrije Universiteit Brussel (VUB) relies on the creation of microvortices within the channel, which ensure that all molecules move (roughly) at the same axial velocity through the column. By generating exclusively laterally oriented vortices in micron-sized channels, we were able to show a reduction in plate height at high velocities by a factor of 3–5.
The how – and why
Why use a vortex for chromatographic separations? Well, in vortex LC you create a chemical system that behaves as if it has an increase in molecular diffusion coefficient, but then only in the direction that is useful to suppress velocity field induced dispersion (so called C-term or related Taylor-Aris dispersion).
There are two important aspects to vortex chromatography. First, higher performance can be obtained in the presence of vortices. Second, it is possible to achieve decent separation performance in channels of a few µm, which, when combined with vortex flows, behave chromatographically as sub-micron channels. With pressures of just a few bars and a low voltage battery to induce lateral flows, we can achieve chromatographic performance that is normally gained using a high-pressure pump in an expert lab environment.
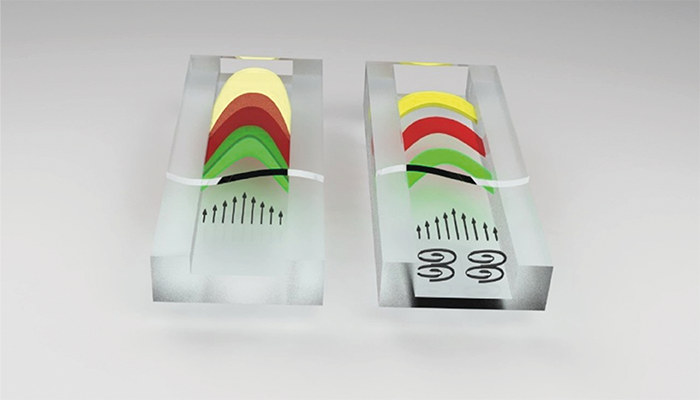
How do we induce vortices? By applying an oscillating electrical potential (below 10 V) across the channel boundaries. This results in a sustained vortex flow, which has as an effect that all species in a chromatographic species band have an average axial velocity with a much narrower velocity distribution. This results in narrower bands, faster separations, and a higher resolution. I have been lucky to be surrounded by many different groups over the years that have opened my eyes to potential solutions – and applications. For example, during my PhD I spent most of my time at the Mesa+ Institute in The Netherlands – an institute that is built around many technology groups where researchers from totally different disciplines are continuously exposed to one another. This has trained me to think and feel comfortable in domains centered around different physics. I have been fortunate to work in several cleanrooms, where I learned the craft of microfabrication, allowing me to propose very detailed process flows for devices that we wanted to conceive – an important skill given the large cost of such devices; you typically only have a single shot because of budget limitations!
Now at VUB, I am again surrounded by top-notch groups active in biotechnology, microbiology, fertility, optics, chemical engineering, diabetes – all areas wherein after intensive discussions we were able to identify a critical hurdle that could be overcome with microfabrication.
Our vortex chromatography approach can be applied in plastic substrates that can be potentially mass-produced at very low cost. And because the columns will operate at low pressure and detection will be integrated in the column, we also expect the instrument size and cost to be reduced in the future. As a first high impact application we will focus on the quantification of glycosylated hemoglobin in human blood for diabetes monitoring. We are currently also pursuing acoustic actuation to conduct vortex chromatography, as an alternative to electroosmotic mixing. It seems that whatever I do, it always ends with a chromatographic application!
Overall, I’ve found joy in the exercise of finding technological solutions to problems, such as the limitations of flow-related methods; and I’m excited to see where we – and others – can take vortex chromatography!
A Clean Start
The group of collaborators, enthusiastic researchers, and developers at my university and professional circle that can benefit from microfabrication is now so large that we have constructed a new cleanroom (MICROLAB core facility) with a focus on ultra-high aspect ratio patterning of glass and silicon devices, mainly for microfluidics. We will develop applications wherein extreme control of structures is essential to enable novel separations. One such potential application is the continuous (production scale) separation of small molecules.
Another area is acoustofluidics, with which we are separating particles, including motile particles like sperm cells. This is another example where the channel dimension after etching should remain within a few percent of the target dimension. As the channel is deeper, the attainable throughput can also be increased, making the method relevant for production environments in the pharma environment, for example.
Headshot - Credit: Thierry Geenen. | Figures - Credit: Wim de Malsche




