The power and flexibility of ion-mobility spectrometrymass spectrometry (IMSMS) enables accurate determination of a broad range of analytes – from small molecules to large protein complexes. The workflow and data quality advantages of IMS-MS instruments like the SELECT SERIES Cyclic IMS are increasingly driving the technology’s use in both high-end research and towards more routine applications. Kevin Giles, Scientific Fellow, MS Research, Waters Corporation, UK, tells us more.
Can you summarize the impact of IMS-MS?
MS is an incredibly powerful technology, which, in combination with IMS, is taken to the next level. Crucially, the extra power is compatible with modern analytical workflows; IMS gas-phase separation timescales sit perfectly between those of LC separation and time-of-flight (Tof) MS, allowing nested acquisitions and high peak capacities. The upshot? Improved data quality and enhanced confidence in results. Furthermore, as IMS sits after sample ionization, the method is particularly valuable for ambient ionization approaches (such as DESI, ASAP or REIMS), MALDI and MS imaging, where upstream chromatography is not possible. Finally, IMS can determine gas-phase collision cross-section (CCS) values of ionized analytes. That’s important because CCS values relate to gas-phase structures and can be used to identify species (through comparison with theoretically-derived CCS values) and as an additional confirmation metric in screening experiments (using CCS libraries). For all these reasons, we are seeing increasing interest in IMS-MS.
How have you responded to this increased interest?
Our recent webinar series (see the sidebar “IMS-MS Webinar Series”) answers many common questions and requests for advice. For example, the “Introduction to Ion Mobility and the SELECT SERIES Cyclic IMS” presentation outlines the basic principles of ion mobility separation and the unique capabilities of the Cyclic IMS-MS system.

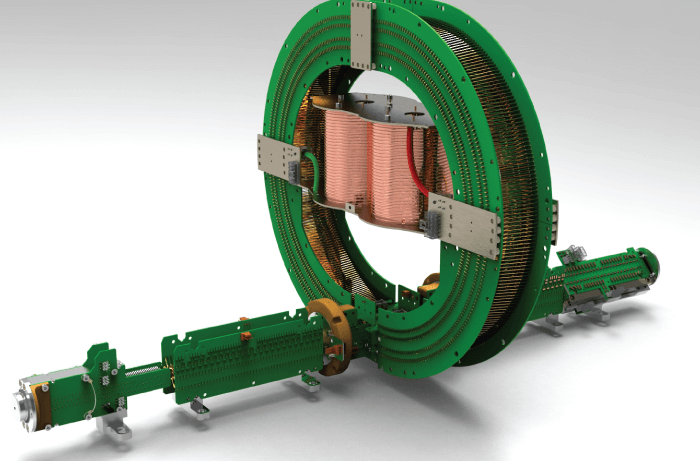
Why was the Cyclic IMS-MS developed?
In short, it’s the natural result of Waters’ constant drive to improve performance characteristics and functionality of our analytical technologies. The Cyclic IMSMS system advantages include increased IM separation capability, multi-functional operating modes (providing more in-depth molecular characterization) seamlessly integrated in an enhanced Q-ToF MS with m/z resolution up to 100,000, mass accuracy down to 500 ppb, increased sensitivity (especially for more labile species), and improved dynamic range. Given all the advantages, I suppose the question should be, “Why would you not develop such an IMS-MS system?”
And what makes the Cyclic IMS instrument unique?
Like Waters’ SYNAPT instruments, it has a Q-IM-ToF geometry (all other manufacturers use an IM-Q-ToF geometry). Crucially, these instruments feature collision cell placement before IM separation, as well as after, which permits separation of fragment ions from m/z-selected precursors and measurement of CCS values to aid structural determination. Importantly, the cyclic IMS allows multi-pass operation, providing userselectable resolving power, which we’ve shown to scale from ~65 (for one pass) to >750 (for 100 passes). Such performance is unmatched in a commercial instrument. But perhaps the most unique feature of the Cyclic IMS is its ability to perform IMSn acquisitions, analogous to the MSn mode in ion traps, which allows structural analysis of unparalleled detail. And users get all these features in a remarkably small footprint!
IMS-MS Webinar Series – View on demand
- Kevin Giles, Waters Corporation - Ion Mobility and the SELECT SERIES Cyclic IMS
- David Ropartz, INRAE - Characterization of oligo-/ polysaccharides
- Joseph Zaia, Boston University - Characterization of glycopeptides
- Libin Xu, University of Washington - Lipid Profiling and MS Imaging
- Ralf Laux, Boehringer Ingelheim and Ian Wilson, Imperial College London - MetID and structural elucidation
- Mark Skehel, MRC LMB Cambridge - HDX/MS Experiments
What main challenges were encountered during development of the Cyclic IMS?
There were a number of challenges in the development of the Cyclic IMS device. One critical issue we faced was related to control of ions at the interface between the main ion optical axis of the instrument and the orthogonal Cyclic IMS device. To address this, we created an electrode array in which ion movement could be changed by switching the direction of the traveling waves used for ion propulsion and mobility separation. This system facilitates ion entry/ exit from the Cyclic IMS, including mobility selected ejection used in IMSn.
Are there any unsung heroes in the development of the Cyclic IMS?
Bringing a product to market involves many people, and the Cyclic IMS instrument is a testament to the skill and creativity of the entire Waters team in Wilmslow, UK. But I would like to give particular mention to Peter Carney, Bharat Chande, John Garside, Paul McIver, and Peter Nixon, who were pivotal in developing the first cyclic IMS prototype back in 2014.
What research has Cyclic IMS facilitated?
Some early work we did with David Ropartz at INRAE provides a particularly interesting example. We used the Cyclic IMS to separate isomeric reducing pentasaccharides; by combining this approach with higher resolution analysis, IMSn and isotope labeling, we confirmed mobility separation of the anomeric forms. The application of IMSn to locate structural modifications in isomeric species is potentially very far-reaching!
What customer feedback have you received?
All very positive! By participating in the Cyclic IMS community, we can see that analysts’ imaginations have been captured by the flexibility of Cyclic IMS operation, both in terms of “dial-up” resolution and IMSn capability. They also very much appreciate the raw performance power of the instrument. Frequently, this translates into the best feedback of all – a decision to purchase a SELECT SERIES Cyclic IMS instrument. People only buy instruments if they believe in their value!
What is the future of IMS-MS?
I think there is a very bright future, both in its growing use as a routine analytical tool, enhancing workflows and data quality, and as a powerful research tool to help unravel some of the most complex analytical challenges. Overall, it will play a central role in progr essing our customers’ science across a wide range of application areas.
IMS-MS Webinar 1 Q&A highlights
Expert panel: Dale CooperShepherd and Kevin Giles
Can Cyclic IMS both separate structural conformers and also isolate/trap them for further investigation?
KG: Yes. The unique geometry of this system permits selection of subpopulations of mobility-separated ions, followed by their activation and reintroduction into the instrument for further mobility separation. We can repeat this sequence in an IMSn experimental design that is analogous to MSn .
What is the duty cycle efficiency of Cyclic IMS?
KG: A crucial feature of our mobility systems is that, while the instruments are performing one mobility separation, they are also accumulating the set of ions for the next mobility separation. And that’s why our duty cycle is close to 100 percent.
What is the role of the step-wave?
KG: It efficiently transfers ions from the source to the mass analyzer. Note that ions are focused off-axis, then transferred to the mass analyzer through differential apertures. The off-axis design moves ions orthogonally to the main flow of the gas – and that helps keep the system clean; any droplets from the ion source simply pass straight through.
What are the main benefits of IMS?
D C-S: When interrogating complex samples, some analytes (including isomers) may co-elute; separation by ion mobility allows us to discriminate between these species. Also, IMS supports measurement of collision cross section (CCS), which can be compared with a CCS library, giving extra confidence in analyte identification – especially for very low-level species.
What fragmentation options are compatible with Cyclic IMS?
D C-S: The standard fragmentation method is collision-induced dissociation, which we can do either before or after the ion mobility step. In IMSn experiments, dissociation can be effected within the Cyclic device between rounds of ion mobility. Last year, we introduced electron capture dissociation – a complementary fragmentation technique frequently applied to protein and peptide studies; again, this can be used before or after ion mobility. Also, we recently implemented surface-induced dissociation (SID), which allows us to dissect the interconnectivity of non-covalent interactions within native protein complexes.
Are multiple pass separation times compatible with LC?
KG: Yes. Typical separations of 2-5 passes take less than 100 milliseconds. And even if you opt for the extreme, say 100 passes, it needs only ~1.5 seconds.
Do prolonged times in the ion mobility region adversely affect resolution?
KG: No. Although ions spend longer in the device, they cannot significantly diffuse radially because they are confined; at the same time though, they are being separated axially –and this step obviously improves the resolving power of the system as increasingly longer drift paths, hence residence times, are used.