I’ve got a challenge for you. Try to think of a significant scientific advancement that was not made possible by the development of a tool that enabled us to see something or measure something (including everything from litmus paper to telescopes). Struggling?
Well, that’s why MS has had such a significant impact in our field. When I was a graduate student in the 1970s, mass spectrometers were large, clunky instruments that were not computerized (see Figure 1) and were typically used to explore fundamentals in physics and physical chemistry. Although many instrumental developments came out of early physics or physical chemistry research, their biggest impact has been in analytical chemistry. As a result of these advances, MS has become the gold standard – the flagship of analytical chemistry – solving problems in an enormous range of applications, from drug discovery to environmental research, testing Olympians and screening newborns for inherited diseases.
You might say this a love letter to MS – and perhaps it is – but, if you’ll allow me, I’d like to look back on the great voyage this technique has been on over the years, from its humble beginnings to the exciting future that lays ahead.

I would identify four major developments in instrumentation that got MS to where it is today. First up: new mass analyzers that were (more easily) computer controlled, faster, and enabled on-line chromatography/mass spec. In particular, the quadrupole mass filter – developed by Wolfgang Paul in the 1960s (1989 Nobel Prize in Physics). However, it wasn’t until the 1970s that the quadrupole found its true calling in computerized GC-MS. This advance was largely driven by the US EPA’s demand for environmental analysis, and achieved by Mike Story and Bob Finnigan at Finnigan Corporation (now Thermo Fisher Scientific). Despite a rough start – it was once called a “toy, not a real mass spectrometer” by a leading mass spectrometrist back in the mid-1970s – the quadrupole is now at the heart of almost every modern MS, whether as a quadrupole mass filter, a quadrupole ion trap, or a quadrupole or multipole ion guide.
The second, but equally important milestone, was the development of tandem MS (MS/MS). But there were a few key steps that needed to be taken first. Although the earliest implementations were with magnetic sector mass analyzers, it was the triple quadrupole that made computer-controlled MS/MS (and GC-MS/MS and LC-MS/MS) practical and commercially successful (first by Finnigan, and subsequently by Sciex, Agilent, and Waters). The triple quad, as something I was personally involved in, will come up again later, but it’s worth noting that the development of low-energy collision-induced dissociation (CID) in an RF-only multipole collision cell was vital to its development. Although most experts in the field said it would never work, it turned out to be far more efficient than high-energy CID in sector instruments – and was ultimately part of the patent. Again, low-energy CID is used in every tandem mass spectrometer today, and MS/MS has become commonplace in analytical chemistry. This is particularly true for LC-MS/MS, as the ionization sources we use generally do not provide any fragmentation for compound identification.
Of course, the triple quad is the accepted standard for targeted quantitation in GC-MS/MS and LC-MS/MS, but what about untargeted (global or exploratory) analysis? In these cases, tandem mass spectrometers employing high resolution mass analyzers that can provide the exact mass for unknown compounds are incredibly helpful. The breakthrough here was the development of high-performance HRMS analyzers, particularly modern time-of-flight instruments (enabled by advances in high-speed electronics) and the Orbitrap (the Fourier transform mass analyzer developed by Alexander Makarov). These new high resolution mass spectrometers have now almost completely replaced the sector mass spectrometers that dominated the MS field 50 years ago.
The fourth key advance that enabled modern MS? New ionization techniques. Historically, electron ionization was the go-to: we would get compounds in the gas phase and then bombard them with electrons. But then electrospray ionization came along, developed by John Fenn and colleagues at Yale University (Nobel Prize in Chemistry 2002), followed by matrix-assisted laser desorption/ionization (MALDI). The development of new ionization methods that could ionize involatile and thermally labile compounds, even proteins and other large biomolecules, opened the door for LC-MS, enabling the separation of compounds that are not volatile and thermally stable enough to make it through a GC column. MALDI was the key to practical imaging MS, which has great potential for combining the enormous detection and identification power of MS with microscopy to image biological tissue. There is no doubt these new ionization methods dramatically expanded the scope of MS, helping drive advances in proteomics and many other aspects of biological and biomedical science.
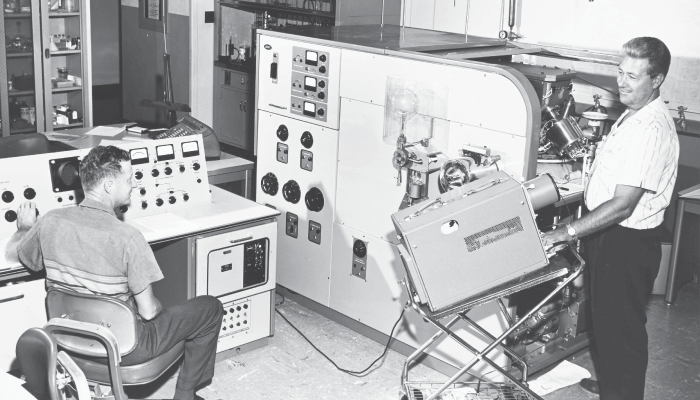
These four key developments stand out to me in the history of MS, but my personal journey with the technique is centered around the triple quad. I first became interested in MS when I started my PhD program 45 years ago. As I said, mass spectrometers back then were large, clunky instruments – and computer control was but a pipe dream! They featured big magnetic sectors and were designed to make measurements in physics or physical chemistry – not analytical chemistry.
Whenever I consider tracing the development of a new analytical instrument or method, I think back to an editorial in the December 1973 issue of the Journal Analytical Chemistry by Herb Laitinen (my educational grandfather) on “The Seven Ages of an Analytical Method.” In his typical insightful fashion, Herb provided a roadmap for the evolution of new instruments and methods:
- Conception of fundamental principles
- Experimental validation of analytical potential
- Instrumental developments/availability
- Establishments of a solid fundamental foundation
- Widened scope of application
- Acceptance as a routine, standard method
- Senescence, overtaken by newer methods
Let’s follow that map for tandem MS…
Ah, the first two ages… The fundamental principles of MS/MS (though that term hadn’t been coined yet) were first shown in the observation of “metastable peaks” (very broad mass peaks at non-integer m/z values) in high resolution mass spectra obtained on sector instruments. Graham Cooks at Purdue and others recognized the potential of these metastable peaks for direct mixture analysis, without prior clean-up or separation.
I was part of the third age. In 1975, I started graduate studies in analytical chemistry at Michigan State University. Because I was interested in the role of computers and electronics in advancing analytical chemistry, I chose to work with Chris Enke, who had a remarkable “big picture” view of the field. But he was (shock, horror!) an electrochemist – and I thought electrochemistry was black magic. Nevertheless, Chris said I could join his group. At one point, I told Chris that I wanted to develop the “ultimate computerized mass spectrometer,” and that it should have a quadrupole in it! So where did that come from? Well, I’d just completed my undergraduate studies at the University of Arizona. In an instrumental analysis lecture, Bonner Denton had passed around a quadrupole, a new kind of mass analyzer that was far more attractive for computer control than a huge electromagnet! The seed was planted.
Driving home late one night from the 1975 FACSS meeting in Indianapolis, Chris and I outlined the concept of a computer-controlled tandem quadrupole MS/MS instrument. Chris suggested I write an NSF proposal, so I did! Our main tact was that it could be used for structure elucidation and mixture analysis. Indeed, we wrote: “The ability to control the acquisition of tandem mass spectral data in real time to answer a chemical question rapidly and with confidence will be a big step toward the goal of the ultimate system for chemical analysis.”
As you may have guessed, the NSF proposal reviews weren’t great:
- “The proposal indicates a serious lack of familiarity with mass spectrometry, and there is little chance that the instrument will produce useful data.”
- “It is doubtful that the proposed instrument offers any real advantages over sector instruments.”
- “Experience with tandem mass spectrometers indicates that computer control is impractical.”
Not dissuaded, we sent a copy of the proposal to the Office of Naval Research, and they ended up funding the project! We bought 2000 pounds of stainless steel and electronics and started carving.
At the 1977 ASMS Conference, Chris and I discussed our ideas with Jim Morrison from LaTrobe University in Australia. He was the first mass spectrometrist to think that the instrument would work! After all, Jim and his grad student, Don McGilvery, had built a triple quadrupole instrument for optical spectroscopy of ions using a tunable dye laser (though they had never managed to obtain a mass spectrum with the instrument). Even at high vacuum (10-7 Torr), they observed more collision-induced fragmentation than photo-induced fragmentation, and that’s what we wanted for our MS/MS experiments. I was able to talk the Office of Naval Research into funding a trip to Australia for two months to perform preliminary experiments, as our instrument at MSU was still under construction. Those experiments led to the first two manuscripts on tandem mass spectrometry with a triple quad and on low-energy collision-induced dissociation, as well as a patent.
I accepted a faculty position at the University of Florida in 1979, planning to build another triple quad (my proposal to NSF was successful this time!). But I was able to work with Finnigan Instruments to produce the first commercial triple quadrupole MS. Although Finnigan estimated that the “worldwide market would be perhaps 10 instruments,” 40 years later the triple quad is the world’s most widely used mass spectrometer, with over $1billion worth of instruments sold each year!
Check out the video, “A look back at the birth of the triple quadrupole mass spectrometer,” for more on this story.
It is interesting to reflect on how and why MS has grown so dramatically in so many different areas, when thinking about the future. Back in my grad student days, optical spectroscopy was the dominant instrumental analytical method. In an optical spectrometer, you put a sample in a cuvette, pass light or other kinds of electromagnetic radiation through it, and then sort out the light. However, in a mass spectrometer, you ionize the molecules, and you separate the molecules themselves. Thus, MS is unique amongst spectroscopic techniques as it not only gives you a spectrum, it also separates the ionized molecules themselves by their mass-to-charge ratio (m/z). This makes MS uniquely powerful for solving complicated problems, which is one of the primary reasons why it has become one of the dominant analytical methodologies of the 21st century.
When I first became a faculty member of the University of Florida 42 years ago, we published a feature article in the journal Analytical Chemistry on the triple quadrupole mass spec. I recently looked back at that issue to see how many of the papers were on MS – it was three out of 50. If you look at the most recent issue of Analytical Chemistry, a third of the papers are on MS – what better way to illustrate the remarkable growth of analytical mass spec!
And I think MS just gets bigger and better from here. It will continue to evolve, both in terms of instrumentation and in the range of applications. In particular, there are two advances that I would love to see in MS over the next decade or two. First, I would like an ionization technique that handles the kinds of thermally labile and involatile biological molecules we work with every day, but that also works as well as electron ionization (EI). EI is both universal and democratic – that means it ionizes essentially all compounds (remembering that they have to be in the gas phase), and it doesn’t matter what other compounds are present – so everyone gets the same vote, no matter who else is voting! Current ionization techniques for thermally labile and involatile molecules do not have either of these characteristics – some compounds are ionized far less efficiently than others, and the presence of one compound can suppress the ionization of another compound. That causes enormous difficulties in LC-MS that aren’t seen in GC-MS using electron ionization.
Second, I would like a chromatographic separation technique for large and involatile molecules to replace or enhance LC – one that works as well as capillary GC, offering phenomenal resolution while being routine to implement. HPLC and UHPLC do not offer the separation power of classic capillary GC, nor are they as simple and reliable. But we use them because they allow us to separate thermally labile and involatile compounds that do not readily make it through a heated GC column (in the gas phase).
Finally, in terms of where MS is going, we have to realize that MS produces enormous amounts of data. Computerization of MS has become increasingly important, and today you need a terabyte hard drive next to your mass spectrometer to take even a week's worth of data.
Having been involved in mass spectrometry for nearly 50 years, I really look forward to seeing what the next 50 will bring us!