It’s an unfortunate fact that the best conditions for chromatographic resolution are those least conducive to high sensitivity in mass spectrometry. Specifically, ion-pairing agents or high salt concentrations are the basis for improved resolution in chromatography – but they are the bane of mass spectrometry. Reversed-phase liquid chromatography with mass spectrometry has arguably been the most successful marriage between HPLC and MS, because acidic modifiers that avoid ion-pairing have been a tolerable compromise. Other separation modes fare worse, including hydrophilic interaction liquid chromatography (HILIC) and hydrophobic interaction chromatography (HIC). Here, we present new strategies that make HPLC-MS work with less compromise in HILIC and HIC modes.
HILIC into shape
HILIC (for the uninitiated, a type of normal-phase chromatography based on hydrophilicity, where the mobile phase is acetonitrile/water and the stationary phase is a hydrophilic layer (1)) has attracted increased interest in recent years due to its ability to characterize protein glycosylation (sugars are very hydrophilic so retention is longer when the glycan has more sugar groups). Why is glycosylation of such interest to bioanalytical chemists? Two reasons: first, many biological therapeutics are glycoproteins and their effectiveness relies on having the same glycan sequence as the native human protein (2); second, most of the human proteome is comprised of glycoproteins, and aberrant glycosylation is a hallmark of cancer (3). Thus, improvements in speed and sensitivity in characterizing protein glycosylation could have a broad impact in medicine. HILIC has high efficiency and compatibility with MS for glycans but lower resolution and less MS compatibility for glycoproteins. The current workaround for characterizing protein glycosylation is to enzymatically release the glycans from the glycoproteins, label the glycans, and perform HILIC-MS. However, this process is slow and labor-intensive, and doesn’t tell us where the glycan is attached. We wanted to improve HILIC resolution for intact glycoproteins, and this led us to make a bonded phase that allows us to use HILIC-MS for the characterization of intact glycoproteins (Figure 1). In Figure 1a, conventional small-molecule bonded phases allow for electrostatic interactions between the positively charged protein and the negatively charged silanols on the silica surface. Our idea? We use a thick polymer layer to create a greater distance between the protein and the silica surface (Figure 1b). Since the electrostatic potential decays exponentially from the surface, distances longer than a few nanometers will screen the charge well. We have shown that this approach gives better resolution of an intact model glycoprotein, ribonuclease B, compared to commercial columns (4). In making the bonded phase, the polymer layers are grown from the silica by atom-transfer radical polymerization. This technology was first demonstrated in 1997, where polymer chains were grown from surface-bound initiators on silica in a controlled way, largely avoiding polymer formation in solution (6).A middle-down middle ground
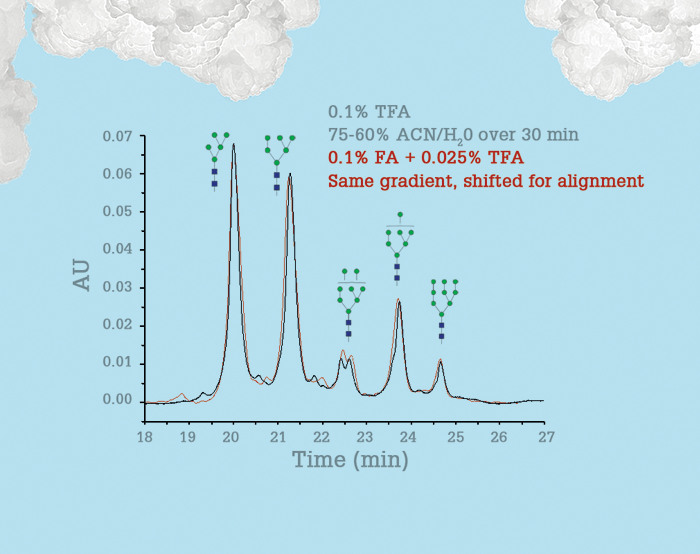
The approach of using a thicker bonded phase allows the use of MS-compatible mobile phase modifiers. Trifluoroacetic acid (TFA) is used as a mobile phase modifier for HILIC of glycoproteins because it is a strong enough acid to neutralize the most acidic silanols on the silica, but it greatly reduces sensitivity in MS because its anion forms adducts with proteins. Formic acid is compatible with mass spectrometry, but resolution in HILIC is lost because the pKa of formic acid is much higher than that of TFA, which makes the silica surface more charged. With a much thicker bonded phase, formic acid can be used with very little TFA because the surface charge is screened by the polymer.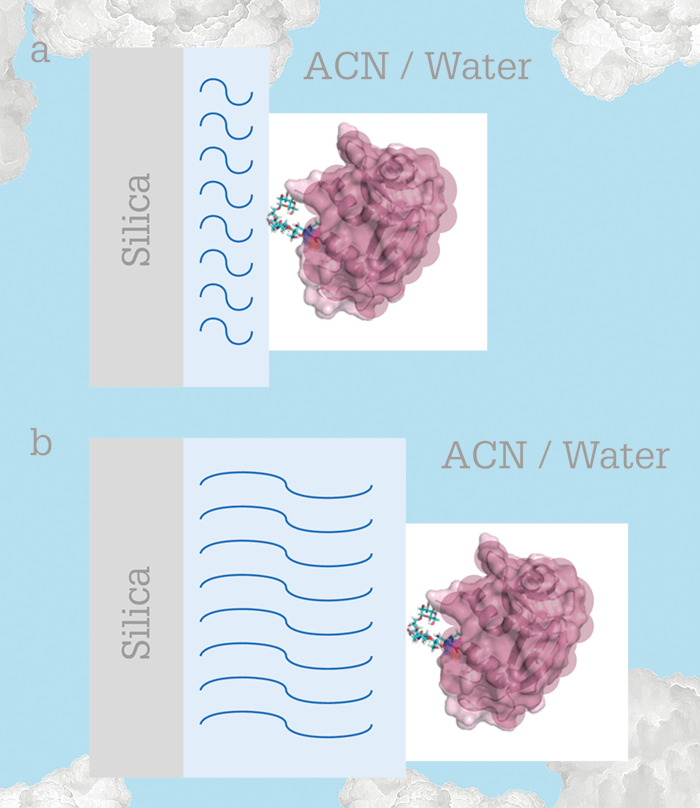
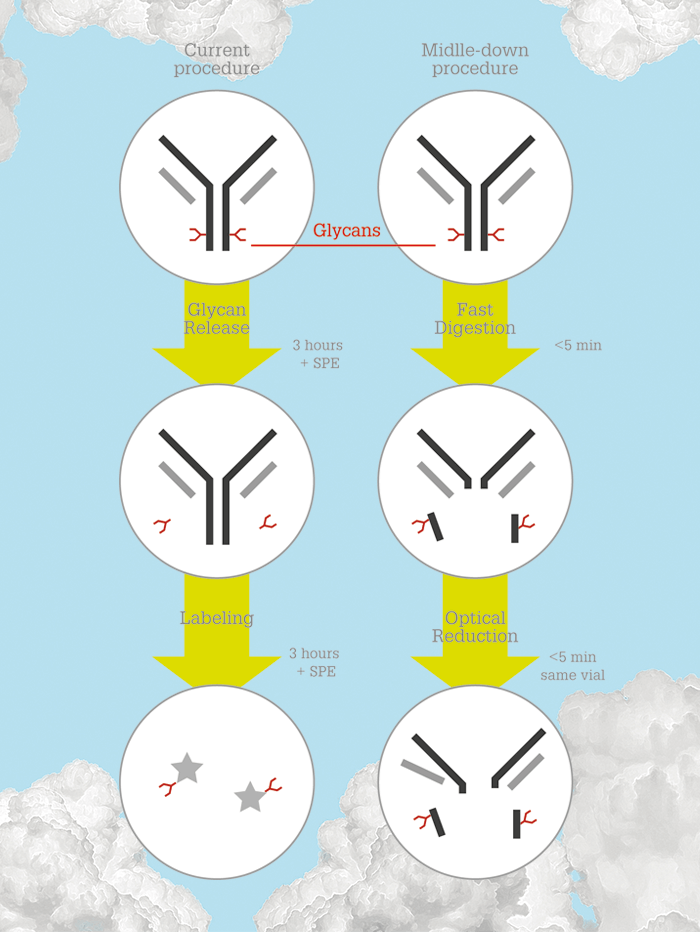
To illustrate how much a polymeric bonded phase improves HILIC-MS, take a look at the chromatograms in Figure 2 for ribonuclease B. The change from 0.1 percent TFA to the MS-compatible mobile phase of 0.1 percent formic acid plus 0.025 percent TFA results in a negligible change in resolution, but means that we can now consider using HILIC-MS on intact glycoproteins instead of the released glycans. We collaborated with Genentech to test a middle-down procedure for characterizing IgG1 glycosylation, which could shorten the entire analysis time from 1.5 days to less than 30 minutes, while avoiding the labor-intensive steps of conventional glycan analysis. In short, we hoped to enable rapid, automated monitoring of glycosylation during manufacturing, allowing for real-time adjustments (the two procedures are compared side-by-side in Figure 3). The middle-down approach is performed in one vial, with fast reactions, and the contents can be directly injected into the column without further sample preparation. Such an approach has been studied previously, but the HILIC separation did not provide sufficient resolution even with the use of TFA (5). Our HILIC columns provide the resolution needed to make this approach work (publication is pending so we can’t share the data here) – and the resolution is maintained for 0.1 percent formic acid + 0.025 percent TFA. In another application, we collaborated with scientists at Abbvie on HIC-MS of a model antibody–drug conjugate. This project might seem like an impossible dream, given that HIC for such drugs currently requires a salt gradient containing a high concentration of sodium ions, but with the lessened electrostatic interactions, high resolution was maintained using low concentration ammonium formate for HIC-MS. We expect to publish on this soon, as well.
From collaboration to commercialization
We have commercialized this technology through bioVidria (www.biovidria.com), located in the Purdue Research Park. The HILIC and HIC columns, as well as RPLC column, are stainless steel, 50 mm × 2.1 mm, packed with 1.2 µm silica particles with the appropriate covalently bonded copolymer for the separation mode. The intellectual property is protected under an issued patent, US Patent 9,758,542, 2017, with international rights pending. Commercializing the technology has been an adventure for us. Why? Academic research is directed toward the future, whereas commercialization has to survive in the present. To illustrate the dichotomy: the chromatographic resolution is better with submicron particles, but the variability of available frits prevents cost-effective production right now. Another illustration of the difference between academic research and commercial practicality? Polyacrylamide works great for HILIC-MS of intact proteins, but the acrylamide monomer is a neurotoxin that would have to be carefully weighed out in large quantities for reproducible and cost-effective bonded phases. Polyacrylamide swells, which gives high back-pressure and makes the particles fight back during high-pressure packing. It also degrades too fast for the customers’ satisfaction. All of these problems matter little in basic research, but weigh heavily when bringing the technology to practice. The learning process has enabled us to develop a safe, cost-effective, high-performing copolymer for HILIC-MS. The most essential factor in commercialization is collaborating with potential customers. Our collaborators at Genentech, Abbvie and Pfizer were valuable in developing our HILIC, HIC and RPLC columns, respectively. We plan to commercialize more column formats in the near term. The current format is 50 mm x 2.1 mm, common for protein chromatography, but many customers want narrower bore columns because these consume less sample and provide more sensitivity in mass spectrometry. We continue to explore new polymers and, on the basic research side, we are using capillaries and fluorescence imaging to understand the gradient elution process better, identifying where the broadening occurs so that we can design columns for even better separations in the future. Mary J. Wirth is W. Brooks Fortune Distinguished Professor — Analytical Chemistry, and Rachel E. Jacobson and Edwin Alzate are doctoral candidates in the Department of Chemistry, Purdue University, Indiana, USA.References
- AJ Alpert, ”Hydrophilic-interaction chromatography for the separation of peptides, nucleic-acids and other polar compounds”, J Chromatogr, 499, 177–196 (1990). A Zhiqiang, “Therapeutic monoclonal antibodies: from bench to clinic”, J. Wiley & Sons: Hoboken, NJ, (2009). A Kirwan et al., “Glycosylation-based serum biomarkers for cancer diagnostics and prognostics”, Biomed Res Int, [Epub] (2015). Z Zhang et al., “Polyacrylamide brush layer for hydrophilic interaction liquid chromatography of intact glycoproteins”, J of Chromatogr A, 1301, 156–161 (2013). V D’Atri, “Hydrophilic interaction chromatography hyphenated with mass spectrometry: a powerful analytical tool for the comparison of originator and biosimilar therapeutic monoclonal antibodies at the middle-up level of analysis”, Anal Chem, 89, 2086-2092 (2017). XY Huang et al., “Surface-initiated radical polymerization on porous silica”, Anal Chem, 69, 4577–4580 (1997). NG Jayaprakash, A Surolia, “Role of glycosylation in nucleating protein folding and stability”, Biochem J, 474, 2333–2347 (2017).




