Some analytical scientists say mass spectrometry can be divided into two eras – before and after the invention of electrospray ionization (ESI); one such person is Adam Trevitt, who leads a laser chemistry laboratory at the University of Wollongong, Australia. In a recent paper (1), Trevitt and his team set out to better understand how neutral molecules become protonated during positive-ion ESI by investigating methods of detecting and separating protonation isomers.
Specifically, they looked at the antibiotic ciprofloxacin, which is known to have two possible protonation sites. Their findings showed that the resulting protomers could be resolved by differential ion mobility spectrometry. Here, Trevitt shares more details of the study.
What were you trying to achieve?
It is almost impossible to overstate the impact soft ionization processes like ESI have had on MS and chemical analysis. Generally speaking, soft-ionization means the process delivers molecules into the gas-phase intact or with limited fragmentation while leaving the molecule with charge (or multiple charges). A common ionization process is protonation or deprotonation of a neutral molecule. Since the site of protonation can affect fragmentation pathways, ion conformation and mobility, as well as ionization efficiency, we were motivated to investigate the ESI process. To do this, we have been developing methods to detect and separate protonation isomers – ions that only differ by the site of protonation.
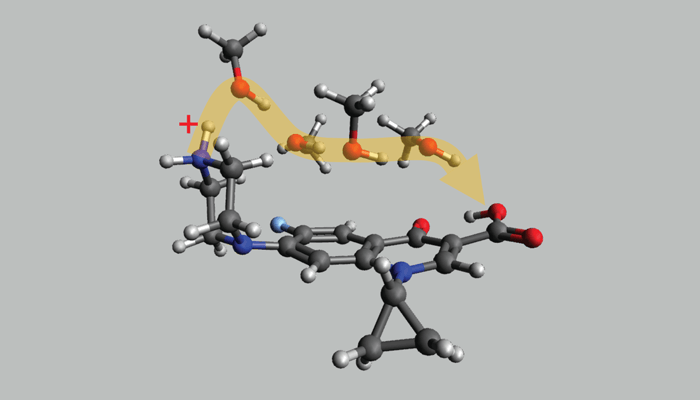
ESI transports molecules from bulk solution to the gas-phase, so we wanted to probe the last steps in this journey in particular – where only a handful of solvent molecules might still be around. Ciprofloxacin, a well-known antibiotic, was a good target as it was already reported to have two major protonation sites, and these can separate under ion mobility. Our idea was to test if we could detect changes in the protonation site when the protonation molecule was exposed to a few solvent molecules – could we gain some mastery over the protonation site populations? To what extent can ESI be manipulated and controlled?
Talk us through your approach…
We used a triple-quadrupole mass spectrometer (Sciex QTRAP 5500) equipped with a planar differential mobility spectrometer (DMS) cell (SelexION), which is situated between the electrospray source and the MS. A small adaptation to the throttle gas line enabled solvent vapor delivery after the mobility cell, which was used to deliver gas-phase solvent molecules to intercept these selected ions. The DMS operates as a filter and can be set to select a target ion population.
Once we proved that the DMS could separate the ciprofloxacin protonation isomers, we could select each isomer and expose it to solvent vapors. This approach allowed us to deduce how each protonation isomer responded to different solvent vapors and track any isomerization.
What are the key takeaways from your research?
One takeaway point from this study is that care must be taken in MS/MS experiments with polyfunctional molecules, as variations in protonation isomer populations can influence the product ion distribution and confound interpretation of fragmentation spectra (particularly, for example, in automated workflows). Secondly, the choice of solvents used in ESI MS experiments can play a large role in the generation and selective suppression of some protonation isomers.
So, specifically in our paper, we showed that methanol solvent can shuttle a proton from one site of the molecule to the other – but this only happens in one direction. The flipside of both of these takeaways is that by exercising control of the protomer distribution using the ESI parameters (such as solvent, temperature, transfer voltages) the user can selectively alter the protomer populations to suit their application – something we want to study further.
What are your next steps?
This study is a good starting point to explore other structurally relevant fluoroquinolones to gain a better understanding into the key driving factors for protomer formation and their propensity for proton isomerism. It also demonstrated the usefulness of the DMS equipped with a gas-source that allows for gas-phase chemistry after DMS selection. We are focused on developing further multi-platform mass spectrometry that combines ion mobility, ion chemistry and laser spectroscopy tools to expand the utility of these techniques and broaden our targets – particularly for disentangling different classes of isomer ions.
References
- B Ucur et al., J Am Soc Mass Spectrom (2022). DOI: 10.1021/jasms.1c00331




