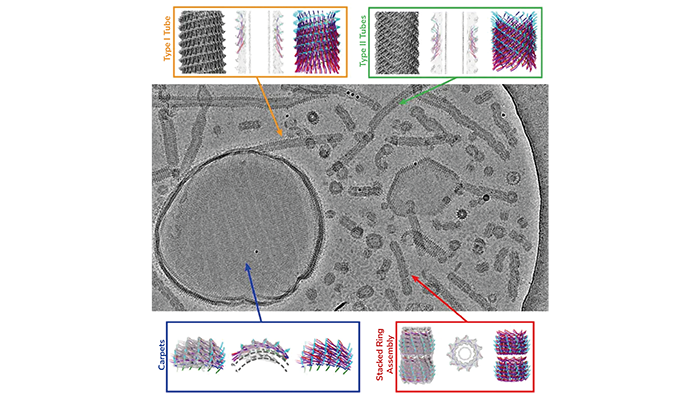
Cryo-EM shows a multitude of different Vipp1 structures: carpet-like structures, ring assemblies and tubes.
Credit: Forschungszentrum Jülich / Bendikt Junglas, Carsten Sachse
New insights into the structural mechanisms through which Vipp1, a membrane protein, repairs and stabilizes cell membranes in photosynthetic organisms have been uncovered by researchers using cryo-electron microscopy (cryo-EM).
Cryo-EM allowed the team from the Ernst-Ruska Centre for Microscopy and Spectroscopy with Electrons, Germany, to capture Vipp1 interacting with membrane surfaces in multiple structural forms. These forms include flat, carpet-like structures that stabilize the membrane surface, as well as ring and tube-like assemblies that appear to remodel damaged or stressed membranes. This detailed imaging provides a clearer understanding of how Vipp1 protects thylakoid membranes, which are essential for photosynthesis in plants, algae, and cyanobacteria.
Vipp1’s ability to form tubes filled with membrane material suggests that these structures may help isolate and remove damaged membrane sections. Additionally, Vipp1’s ability to form ring structures could facilitate the joining of membrane segments, stabilizing the overall structure of the membrane during stress.
In addition to the structural images, the study emphasized the importance of the protein’s α0 helix, which anchors Vipp1 to the membrane. Experiments showed that when this helix was truncated, Vipp1 lost its ability to interact effectively with the membrane, further highlighting its role in membrane protection.
The findings could have broader implications beyond photosynthetic organisms; for example, understanding how proteins like Vipp1 maintain membrane integrity under stress could inspire new approaches in biotechnology and medicine, particularly in the development of biomaterials or treatments for diseases involving membrane damage.
"This research provides valuable insight into how cells maintain their membrane structures, a process that is critical for many biological functions," the researchers concluded.




