© Agilent Technologies, Inc.
Reproduced with Permission, Courtesy of Agilent Technologies, Inc.
If there ever can be a silver lining to a major pandemic, for COVID-19 it was belated but widespread recognition of the power of oligonucleotides. (Did you get a shot of mRNA-based vaccine?). But the history of oligonucleotide medicines is longer than you might think, with some players now leveraging decades of experience in a suddenly very exciting and well funded area.
Here, we speak with oligonucleotide guru Ingo Röhl – Managing Director Analytics, Axolabs, Germany.
How did you find yourself in the oligonucleotide space – before it hit the “big time?”
I stepped into the oligonucleotide field right after my PhD on natural compound analysis, working for a German biotech company in Berlin called NOXXON Pharma (now calle TME Pharma). They developed “Spiegelmers” and I was responsible for building up the analytical lab – an exciting challenge that piqued my interest despite no previous experience with oligonucleotides. After three years, I moved to Ribopharma (the first company to really focus on therapeutic RNAi) just as it was merging with Alnylam – another RNAi-focused company with significant venture capital funding. We were Alnylam Europe for four years or so, developing analytical methods to characterize materials. But as part of a biotech licensing deal in 2007, the German part of Alnylam became the RNA Center of Excellence within the Roche organization. For me, that means a move from biotech to big pharma!
In the 2010s, Roche and other big pharma companies exited the oligonucleotide space, and the team here in Kulmbach decided upon a management buyout. Our ambition was to become a specialist contract research organization – applying all the knowledge we had gained in the oligonucleotide space over the previous 10 years. In short, Axolabs was founded in 2012 – and we’re still a specialist in the oligonucleotide field, but now, thanks to additional investment on becoming LGC Axolabs, we’ve significantly expanded (from 20 to 200 people!) and now offer a “one-stop-shop” for oligonucleotide- and mRNA-based therapeutics – from bioinformatics and lead ID, across all clinical stages, to commercial supply, via cGMP manufacturing facilities.

Looking back to the early days, what were the main challenges you faced as an ambitious analytical chemist moving from small molecules to oligonucleotides?
When I started in early 2000, LC-MS was not so common – and nor was the introduction of UHPLC. There were well established HPLC-UV methods for oligonucleotides up to 20–50 nucleotides in length, but without mass spectrometry, there were many unknowns – co-eluting impurities that could not be resolved, for example. Back then, we had to focus a lot on the HPLC separations, which was challenging but also very interesting.
Over time, the entities that were being pursued as therapeutic modalities became increasingly complex. In the early 2010s, we saw the arrival of mRNA on the horizon thanks to the likes of Moderna and BioNTech – and suddenly we were talking about 1000s of nucleotides rather than 20. In 2013/14, the CRISPR/Cas single guide RNAs entered the picture.
Over the last 20 years or more, many analytical challenges have been resolved by the increasingly powerful combination of HPLC with mass spectrometry. But we still find challenges and open questions that demand new or more advanced analytical tools. For example, deamination makes a difference of just 1 Da, which – even with high-resolution MS systems – can be tricky. In these scenarios, orthogonal methods are often needed to achieve an optimal purity profile.
The success of mRNA vaccines resulted in an explosion of interest – how have you perceived this change as a long-time oligonucleotide specialist?
Certainly, the field has grown! I remember the TIDES Europe conferences in the early 2000s, with around 150–200 people – and I knew most of them. But the field hasn’t just grown, it has changed. The biggest change for us is working with newcomers to the field, who view mRNA as a platform technology, for example, so they are less familiar with the complexities of oligonucleotides; instead, they have invested more time and energy into advanced target knowledge.
Beyond mRNAs, the field has seen other (admittedly less well publicized) successes; we now have approved aptamers, siRNAs, antisense oligonucleotides, splice oligonucleotides, and – most recently, the first CRISPR/Cas entity. Exciting times!
How will the field evolve from here?
I’ve been in the field for 23 years now and I’ve worked on most if not all of the modalities and technologies in this space. In that time, I’ll note that the field has received three Nobel prizes. But what’s really great is that we see approved therapeutics delivering on the promise of those Nobel prizes.
Nobels plus promise – well, that results in solid validation of a platform technology. And that brings investment and yet more excitement. Big pharma is now on board – and we’re seeing oligonucleotides move into blockbuster territory; no one could have foreseen oligonucleotides competing with blood-pressure drugs or statins for cholesterol, for example! We will see a constant flow of funding into this space based on this alone.
At the other end of the spectrum, we also know that these platform technologies enable a move into personalized medicine; we can develop a drug for a single individual based simply on genomic data.
And how will the analytical landscape shift as oligonucleotides take over the world?!
We must prepare for further complexity! For example, as we try to tackle new target tissues, we will need new ways of delivering oligonucleotides – perhaps through conjugation with ligands, the use of vesicles (like the lipid nanoparticles we’ve heard so much about) or exosomes, or vectors. The point is that, despite oligonucleotides themselves benefitting from well-established platforms, increased complexity will come in the form of advanced formulations – and this will bring many new analytical challenges. For example, by simply adding cholesterol to an oligonucleotide, you completely change its biophysical properties – and that means you change its analytical properties.
Looking further ahead, I can imagine generics entering the space, which would demand a great deal of effort in proving comparability – and a lot of questions about stereochemical composition. Here, LC-MS may need to be supplemented by orthogonal techniques like NMR.
One thing is certain, I see no change in the need for analytical scientists who can come up with inventive solutions to fresh challenges!
Welcoming the New Kid on the Block
Like ADCs, oligonucleotides – and the method of delivering them safely and efficiently into cells – pose new analytical questions. Once again, we’re joined by Agilent’s Andreas Mielcarek, who explores current technology and the developments most likely to appear on the horizon.
Oligonucleotides, with their crucial role in therapeutic interventions and genetic research, represent a significant area of focus within modern biopharmaceutical analytics. This guide, distilled from a selection of Agilent’s Application Notes, equips beginners with a practical understanding of the analytical techniques essential for oligonucleotide analysis. Given their complexity – thanks to diverse modifications and the imperative of stringent quality control – oligonucleotide analysis demands advanced and precise analytical methodologies.
Ion Pair Reversed-Phase HPLC (IP-RP-HPLC)
IP-RP-HPLC is a go-to method for separating oligonucleotides based on hydrophobicity. Using ion-pairing agents enhances the retention of charged molecules on reversed-phase columns. It’s particularly effective for characterizing oligonucleotides with modifications, providing insights into purity and composition.
Anion Exchange Chromatography (AEX)
AEX separates oligonucleotides based on charge differences. This technique is invaluable for purifying synthetic oligonucleotides, distinguishing full-length products from truncations and other synthesis-related impurities. Optimizing buffer conditions, such as pH and ionic strength, is crucial for achieving high-resolution separations.
HPLC with UV/Vis Detection (HPLC-UV/Vis)
HPLC-UV/Vis offers a straightforward approach to quantifying oligonucleotides by detecting specific UV absorbance. It’s particularly useful for routine analysis, providing a quick and reliable measurement of concentration and purity without the need for complex sample preparation.
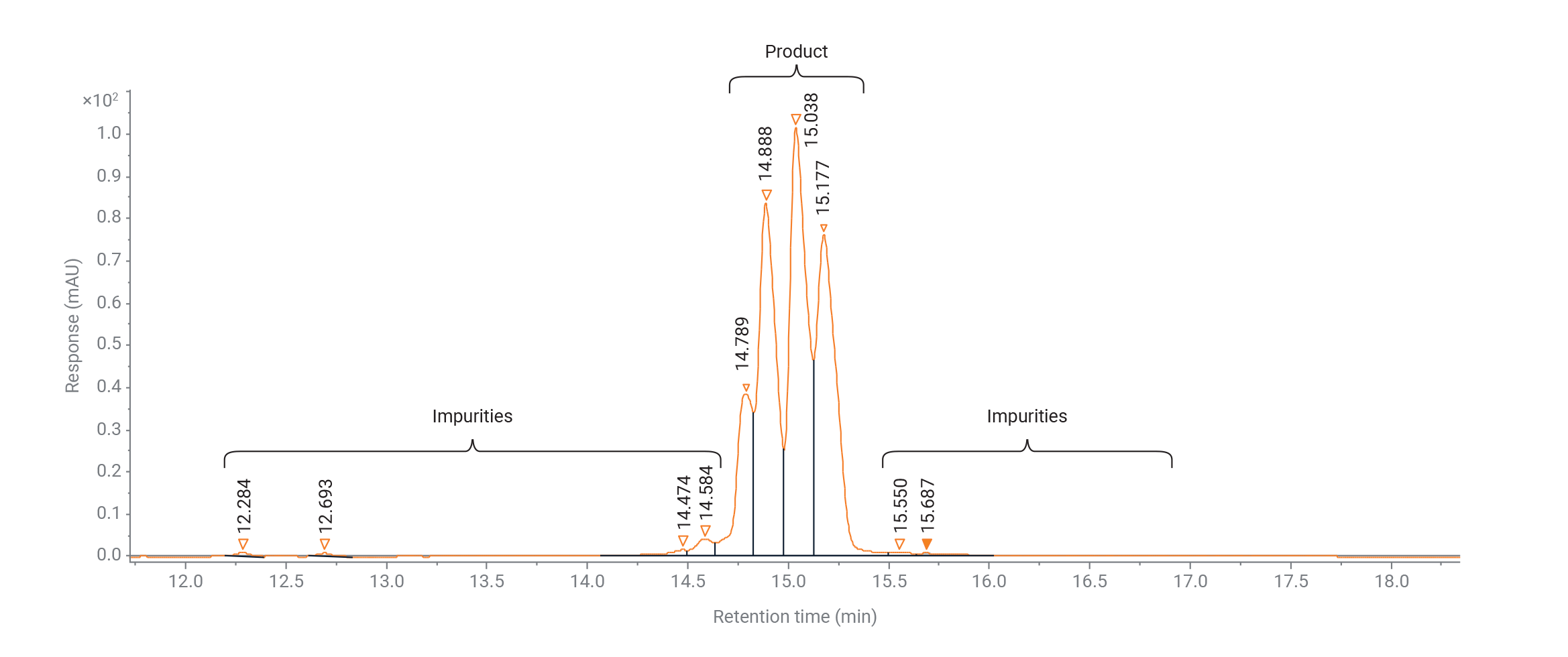
Figure 1. Product and impurities of an siRNA oligonucleotide analyzed with an Agilent 1290 Infinity II Variable Wavelength Detector (VWD) and an Agilent Thermal Equilibration Device (TED).
© Agilent Technologies, Inc.
Reproduced with Permission, Courtesy of Agilent Technologies, Inc.
HPLC with Fluorescence Detection (HPLC-FLD)
HPLC-FLD enhances sensitivity and specificity in oligonucleotide detection by exploiting the fluorescence properties of labeled oligonucleotides or their interactions with fluorescent probes. This method is essential for studying binding interactions and for assays requiring high sensitivity.
HPLC Coupled with Mass Spectrometry (HPLC-MS)
HPLC-MS combines the separation capabilities of HPLC with the precise mass detection of MS. It’s indispensable for identifying oligonucleotide modifications, understanding molecular structures, and confirming molecular weights. This technique is crucial for in-depth characterization and verification of oligonucleotide sequences.
UV-Vis Spectroscopy
UV-Vis Spectroscopy is fundamental for assessing oligonucleotide stability through melting temperature (Tm) analysis. By monitoring absorbance changes at specific wavelengths as temperature increases, it provides insights into the stability of nucleic acid duplexes and the effects of modifications on duplex formation.
Capillary Electrophoresis (CE)
CE offers high-resolution separation of oligonucleotides based on size and charge, using a small amount of sample. Its ability to analyze oligonucleotides rapidly and efficiently makes it a preferred choice for purity assessment and quality control in a high-throughput setting.
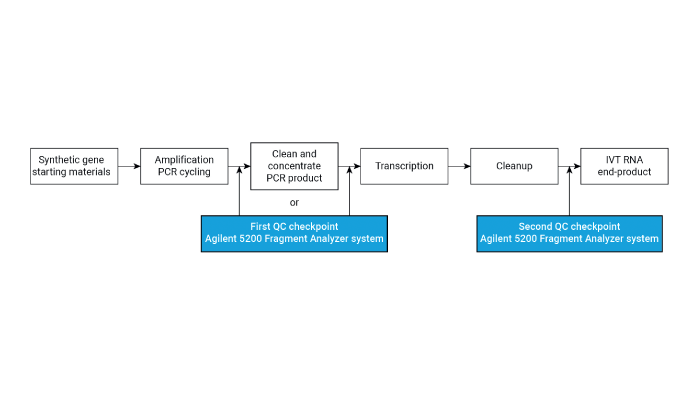
Practical Tips for Getting Started
1. Mastering technique selection. Choose the analytical method based on the specific information needed. For instance, use IP-RP-HPLC for purity assessments, AEX for charge-based separations, HPLC-MS for structural analysis, and UV-Vis spectroscopy for stability studies.
2. Optimizing conditions. Each technique requires careful optimization of conditions. For HPLC, focus on mobile phase composition and column choice. In CE, buffer composition and capillary coating can significantly impact performance.
3. Understanding sample preparation. Proper sample preparation is key to successful analysis. For MS analysis, ensure samples are free from salts and other contaminants that can suppress ionization.
4. Analyzing data with precision. Develop the skills to interpret data accurately – whether chromatograms in HPLC, mass spectra in MS analysis, or melting curves in UV-Vis spectroscopy.
5. Staying informed. Oligonucleotide analysis is a rapidly evolving field. It’s vital to stay current by reviewing the latest literature, participating in workshops and collaborating with peers. Furthermore, using Agilent’s extensive collection of application notes enriches understanding of new techniques and applications, making it an indispensable resource for those seeking to keep pace with advancements and practical methodologies in oligonucleotide analysis.