NASA’s Mars 2020 Perseverance rover landed in the Jezero Crater on Feb 18, 2021. Part of its mission: to search for signs of life and to explore the planet’s geology.
The rover will characterize the planet’s ancient climate and geology, paving the way for human exploration of the Red Planet. It will also be the first mission to collect and cache Martian rock and regolith (broken rock and dust). Subsequent missions, currently under consideration by NASA in cooperation with ESA, will then send spacecraft to Mars to collect these cached samples from the surface and return them to Earth for in-depth analysis.
Fast-forward one year. The ExoMars programme 2022 mission plans to deliver a European rover, Rosalind Franklin, and a Russian surface platform, Kazachok, to the surface of Mars to continue the search for life (or signs thereof) by collecting soil samples with a drill and then analyzing those sample using next-generation instruments – no need for return to Earth! This task is a very special – not only because it may provide answers to some of mankind’s greatest questions, but also because ExoMars will be the first mission to combine our ability to move across the surface of Mars and to study the ground at depth.
A common question at this stage is typically: “What are you looking for in the soil?” Well, we’re not expecting living organisms or burrowing green men – that’s for sure. One apple of our eye is sulfates. These minerals have already been discovered on Mars and act as reservoirs of organic remains and past microbial life.
Juan Manuel Madariaga
I have worked as a Professor of Analytical Chemistry at the University of the Basque Country in Leioa, Spain, since 1993. I’m proud to lead a research group of 34 researchers, 16 of which are permanent staff members. Together, we play a valuable role in the European Network for Research in Heritage Science. My research has focused largely on environmental issues around cultural heritage assets (archeology, artworks, and buildings) as well as extraterrestrial materials. In both of these arenas, I lend a significant focus to the development of analytical procedures and instruments to overcome new challenges.
I’m also the coordinator of the Spanish Network for Geochemical Studies of Mars, which includes five Spanish research institutions. As part of this role, I have the pleasure of contributing to research teams supporting both of the upcoming missions to Mars! At the moment, researchers in my lab are conducting experiments that simulate the processes that result in the formation of different sulfates. The goal: to identify the sulfate compounds that can be found on Mars, and then work with specialists in geophysics and geochemistry, hydrogeology, and sedimentology to propose the processes that may have given rise to them.

The most advanced research on Mars to date was conducted by the Curiosity Rover, which has worked tirelessly since it landed in 2012. One of the instruments on the Rover (an X-Ray diffraction instrument) has reported the presence of three calcium sulfates: gypsum, basanite and anhydrite. Coupled with other findings, such as pyroxenes, olivines, clay minerals, and hematite, but – most importantly – other sulfates like jarosite, these discoveries suggest that Mars once had a wet environment (around 3.8 billion years ago, to be precise). This is another key indication that the planet was once capable of supporting life.
Looking more closely at the elements in these minerals is the next step towards understanding how some of these calcium sulfates were formed. Simple sulfates of this kind are found on Earth (along with other mixed sulfates), but does this mean that Mars once enjoyed similar conditions to those in which life flourishes here?
The general scientific consensus is that geochemical activity on Mars and Earth were similar at one time. In fact, when Mars harbored liquid water billions of years ago, its temperature is also estimated to have been between -10 to +30–40 °C. Sound familiar? Considering both these aspects, it may come as no surprise that we expect to find further mixed sulfates on Mars that can also be found here on Earth.
These shared qualities also mean we are able to identify the places where biosignatures are most likely to be preserved on Mars, based on our searches for similar compounds on Earth. On Mars, this location is the Jezero Crater. The landing and research site for Perseverance, this crater is one of the oldest on Mars and the remnants of a lake from over 3.5 billion years ago. Given that a river once flowed into this lake, it’s the perfect site for the sulfate search.
And, with new instruments (Raman spectrometers) making their way to Mars on Perseverance and Rosalind Franklin, researchers will be able to identify sulfates with more accuracy and power than previously possible. The Raman spectrometers aboard the Perseverance can analyze samples remotely (the remote sensing instrument SuperCam can analyze at distances up to 5 meters) and mere centimetres away (via the SHERLOC, aka Scanning Habitable Environments with Raman and Luminescence for Organics and chemicals, spectrometer), while the Raman laser spectrometer aboard Rosalind Franklin will analyse drilled samples at the 50-micron scale, allowing it to identify both crystalline and amorphous mineral phases. These nifty instruments will also be making their way to the Moons of Mars in upcoming missions – so keep an eye out for that.
Duncan Stacey
I gained my PhD in Optics and Spectroscopy from the University of Liverpool, UK, in 1993. I have worked with scientific instrument manufacturers for microscopy and spectroscopy ever since. Since 2014, I’ve acted as a Sales and Marketing Director for Linkam Scientific Instruments, where I spend much of my time in conversation with leading companies in imaging, spectroscopy, and microscopy to develop our products. I am focused on the development of new markets and research solutions for temperature and environmentally controlled experiments. I am also a fellow of the Royal Microscopical Society and a member of their corporate advisory board.
Our research, which we presented at the virtual Europlanet Science Congress (EPSC) 2020 (1), informs experiments at home (on Earth) that aim to understand the geochemical processes that lead to the formation of key minerals – including sulfates.
As part of this, we synthesize pure minerals to facilitate access to high-quality standards materials for Raman and infrared. These reference spectra can then be used to interpret the unknown Raman and infrared spectra delivered by the instruments onboard Perseverance. Furthermore, these materials will also help in designing protocols to characterize the samples taken by Perseverance when they return to Earth (in approximately 2028–30).
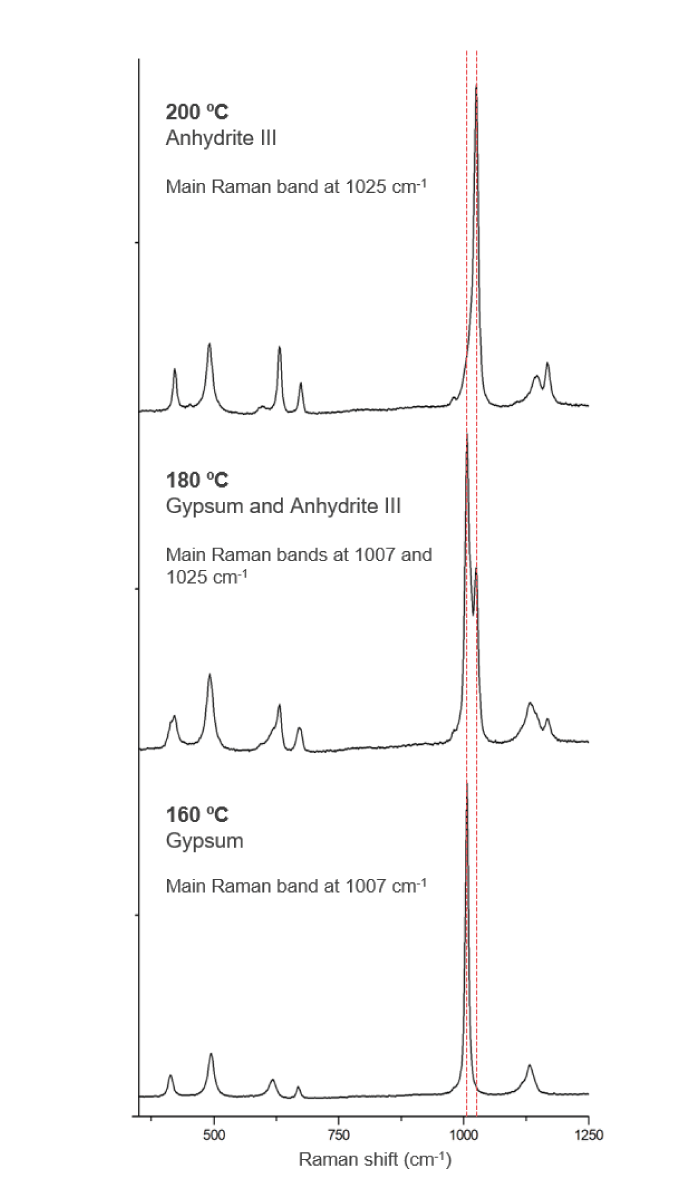
Building on prior research where we investigated the behavior of sodium and potassium sulfates, we performed Raman analyses of three sulfates, one known to be present on Mars – gypsum [CaSO4·2H2O] –, and two other mixed potassium-calcium sulfates – syngenite [K2Ca(SO4)2·H2O] and görgeyite [K2Ca5(SO4)6·H2O] – using a micro-Raman spectrometer with a 532 nm excitation laser and a highly sensitive CCD detector with a mean spectral resolution of 1 cm-1. The spectrometer was also coupled to a temperature-controlled stage for automatic temperature control because of the ability of these sulfate minerals to contain crystallized water; the Raman responses of compounds are sensitive to decreasing and increasing temperatures.
Overall, we tested temperatures ranging from -100 to 400 °C (using a temperature control chamber attached to the Raman spectrometer), allowing the minerals to restabilize after each 20 °C shift (see Figure 1). This range was chosen as these temperatures cover the range we might expect on Mars; the temperature on the planet itself ranges from around -100 to 20 °C, but meteorites ejected from Mars can reach up to 400 °C. As was expected, the tested temperature changes were apparent in the vibrational modes (indicative of molecular changes) in the minerals. More specifically, temperature increases caused a shift in the Raman bands from lower to higher wavenumbers, indicative of our hydrated sulfates becoming anhydrous. Conversely, dropping the temperature did not move the bands, but changes in the form of the bands was observed. This suggests that minerals were not transformed, but rather the crystallinity in the water molecules of the hydrated minerals increased. This information will help us to differentiate between sulfates on Mars – and it could help us to predict the minerals present in meteorites from Mars prior to their ejection from the surface.
Such Raman analysis can also be used to study other types of mineral to gain information about, for example, ancient buildings. Research into mixed sulfates at the Department of Analytical Chemistry at the University of the Basque Country focuses on cultural heritage – in particular, stone buildings (2).
Many different sulfates have been detected in buildings. Typically, these are the result of chemical reactivity between compounds in the atmosphere – sulfuric acid, for instance – and the alkaline compounds common in stone-built walls. The presence of these sulfates, along with nitrates, mean that the walls are – in essence – “washed” when it rains, leading to loss of material. The newly revealed layer beneath can then be attacked by the chemicals in the atmosphere like the previous layer, leading to eventual loss of more and more material.
The problem is clear – and it is a particular challenge in areas close to the sea or industrial ports. The impact of temperature on this process needs to be considered, too. In parts of central Europe, wall temperatures can range from -10 °C in winter to 55 °C at the height of summer, changing the hydration form of soluble sulfate salts present in the efflorescences (the salt deposits left behind by water on stone surfaces) and subefflorescences. Changes from hydrated to anhydrated minerals is also accompanied by changes in volume; for example, mirabilite (Na2SO4.10H2O) has 5 times the volume of thenardite (Na2SO4). As such, these transformations also generate a physical stress in the pores of walls, promoting the formation of cracks and eventually detachments. Our studies on the Raman response of mineral transformations are contributing to a database of enormous importance when it comes analyzing the nature of efflorescences and predicting the associated risks for buildings.
The lab is now on a mission to continue synthesizing stable mixed sulfate minerals to cover the whole scope of mineral candidates that we might expect to find on Mars. And what comes after that? Well, we will continue synthesizing and analyzing other minerals, but this time focusing on perchlorate and chlorate compounds. The hope here is that this knowledge will help us (along with geophysicists, geochemists, sedimentologists, and so on) to elucidate the inner working of Mars’ chlorine cycle.
Though it may sound strange, our research on Mars mineral candidates will also be applied in our stone building research. Why? Because some of the minerals we expect to find on Mars will appear in buildings affected by extreme environmental conditions. Such findings have already been confirmed over the past few years.
Improvements in temperature-controlled and environmentally controlled Raman spectrometers with better detection capabilities also promise a bright future for our research, including (but hopefully not limited to…) high-resolution Raman images and the simulation of extra-terrestrial atmospheric conditions, such as temperature, chemical composition, pressure, and humidity. We are now working on a custom stage that we hope will allow us to recreate the environment on Mars, providing further support to our research. Such capabilities will aid our mission to detect minor and trace mineral phases and their spatial distribution, increasing our chances of predicting where we can detect biosignatures, organic molecules, or water reservoirs. All of this information is invaluable in our hunt for knowledge about habitability – and the search for alien life.
References
- J.M. Madariaga et al. “Temperature transformation of calcium and potassium Martian sulfates as seen by an Exomars 2022 RLS-like Raman instrument” EPSC Abstracts 2020, 14, EPSC2020-1063. https://doi.org/10.5194/epsc2020-1063
- N. Prieto‐Taboada, S. Fdez‐Ortiz de Vallejuelo, M. Veneranda et al. “The Raman spectra of the Na2SO4‐K2SO4 system: Applicability to soluble salts studies in built heritage” J. Raman Spectrosc. 2019 50:175–183. https://doi.org/10.1002/jrs.5550




