A key challenge in adeno-associated virus (AAV) production is the presence of capsids that lack the required gene of interest, which can reduce the overall effectiveness of the therapy. In a recent study, a team of Pall researchers used a novel anion exchange chromatography elution method to separate empty and full AAVs. We spoke with Adam Hejmowski, Team Leader in Bioprocessing Analytics at Pall, and lead author of the study, to find out more.

What are the consequences of not effectively separating empty and full AAVs?
We have to remember that empty AAV particles are duds – they do not carry the therapeutic agent. Having an excess of these duds means the treatment would require higher titers and/or larger injection volumes to guarantee the effectiveness of the treatment, as well as having the potential to cause a larger immune response. In addition, leaving the empty/full ratio undefined leads to product inconsistency and thus varied patient responses – which can be detrimental to the entire gene therapy development process. The importance of the issue led us to consider the need for a defined ratio of empty and full particles for optimal effectiveness.
What are the main challenges?
When attempting to separate any two or more components, challenges often relate to the mode of separation and noteworthy differences between the components. For AAVs in particular, there are typically major differences in Mass (5 MDa full vs 3.7 MDa empty), density (1.4 g/cm3 full vs 1.3 g/cm3 empty), charge/isoelectric point (~5.9 full vs ~6.3 empty, serotype dependent). But these differences are quite small, necessitating specialized, scale-limiting methods, such as ultracentrifugation. In our paper, we focused on the charge differential, using ion exchange chromatography to separate empty and full capsids in a scalable way.
What major challenges did you have to overcome?
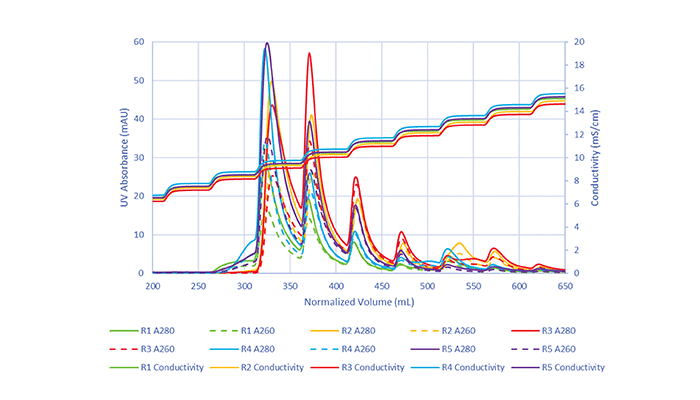
Our initial attempts involved using linear gradient methods with multiple chromatographic formats including membranes, monoliths, and resins. We were disappointed to see that none of the chromatography formats performed as well as we had hoped, so we had to reconsider the method. We needed to tease out more separation and ideally generate a series of discrete elution peaks to be able to judge more easily if the separation was working. From there, we attempted alternative methods, involving linear and step gradients, and modified buffer compositions. We ultimately discovered that simulating a linear gradient via small discrete conductivity steps showed promise in separating empty and full peaks, specifically when using Mustang Q membranes. The coupling of these small conductivity steps with membrane chromatography is where we identified initial and robust success.
In the end, were you satisfied with the results?
We were ecstatic to see the small conductivity step-approach begin to split what originally were small broad peaks into sharper, defined elution peaks. As we performed additional runs of the method and fine-tuned the buffer compositions for our AAV5 constructs, we were satisfied with what was achieved. Using these discrete conductivity steps with membrane chromatography has enabled these successes with AAV and may be highly impactful for gene therapy manufacturing and production.
What implications could this research have for the gene therapy field?
Scalability to manufacturing and larger batch processing has continued to be a struggle faced by many independent companies, as well as larger organizations like Pall. This small conductivity step method provides an approach to easily enrich full AAV particles. Alternatively, a simpler chromatography method could be developed by performing this approach at a smaller scale. Having this framework available to baseline your platform’s polishing steps can allow empty and full AAV separation when the prospect may not have been possible.
Any next steps for this work?
Although satisfied with our method development work so far, we are striving to further improve the technique and showcase its functionality and scalability. We have presented results using this technique to our customers and clients for AAV5, AAV8, and AAV9; and plan to expand our breadth of data to additional serotypes. While in the article we ended with the method using a 5mL Mustang Q capsule, we are confident this method will be applicable to Pall’s larger capsules (50 mL, 140 mL, 450 mL, and 5000 mL); we hope to have more data in the near future.
References
- AL Hejmowski et al., Biotechnology Journal (2021). https://doi.org/10.1002/biot.202100219




