Demystifying Two-Dimensional Liquid Chromatography
Moving from 1D to 2D liquid chromatography is a big step towards the high peak capacities demanded by complex sample analysis.
sponsored by Agilent Technologies
Embracing the Second Dimension
In the past, the separation performance of a chromatographic system was described in terms of column efficiency (N). In liquid chromatography (LC), this value depends on the particle size (dp) and on the column length (L). Using porous particles, N=L/2dp. In 2006, superficially porous particles were re-introduced and the experimental plate number with state-of-the-art instrumentation approached L/1.5dp because of the fast mass transfer in the thin porous shell. Also around a decade ago, tremendous improvements were made in column technology with sub-2 µm porous particles. These, along with improved LC instrumentation that could withstand pressures of up to 1200 bar, opened new possibilities in terms of speed and resolution to LC practitioners.
For today’s complex analyses, plate count is not an effective measure of performance; a better – and now well-accepted – alternative is peak capacity, nc. Introduced by Giddings in 1967 (1), nc is the maximum number of peaks that can fit side-by-side between the first and last peak of interest with a fixed resolution (normally 1). Originally introduced for isocratic separations, Horvath and Lipsky (2) were the first to realize that much higher peak capacities can be attained by gradient elution in LC or temperature programming in GC. The equation generally used to calculate the peak capacity in gradient elution is nc = 1 + tg/W (3) in which tg is the gradient run time and W is the average peak width (4 s).
Before the introduction of high-pressure instrumentation and small particles, peak capacities up to 200 could be obtained in conventional unidimensional LC (1D-LC). Presently, with sub-2 µm porous particles (or superficially porous particles), peak capacities of 570 in 50 min and up to 850 in 180 min have been reported (4). The peak capacity productivity (peaks/min) of a column can be optimized by fine-tuning the gradient time and/or flow to the complexity of the sample.
It is, however, wishful thinking that such peak capacities are sufficient to separate the very complex mixtures that make up biological, food, environmental or natural product samples. Peak capacity should significantly outstrip the number of components in a sample; statistical theory of peak overlap (5) tells us that peak resolution is severely compromised when the number of components present in the sample exceeds 37 percent of the peak capacity. Indeed, to resolve 98 percent of randomly distributed sample components, peak capacity should exceed the number of components by a factor 100 (6). This means that an nc value of 10,000 (corresponding to around 1x108 theoretical) is needed to “chromatographically” resolve a sample containing 100 components! Fortunately, chromatography is not the sole contributor to unraveling sample complexity; the selectivity capacity of contemporary mass spectrometers (MS) substantially lowers the separation-side bar for many applications. However, even with the most powerful MS instruments, maximizing the resolution at the front-end is important, and in QA/QC laboratories where high peak capacities are often needed but mass spectrometers are not yet established, it is essential. One straightforward approach to increasing nc is multidimensional LC, especially bidimensional or 2D-LC.
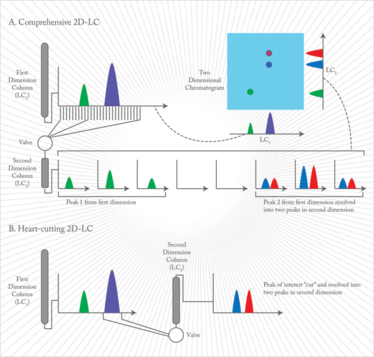
In comprehensive 2D-LC, the complete effluent from the first column is transferred to the second column with a switching valve. The valve transfers the eluent in small portions that are analyzed with fast gradients of 20-30 seconds. After data acquisition, the partial chromatograms of both dimensions are aligned. In heart-cutting 2D-LC, only selected parts of the first column’s effluent are transferred to the second column. By cutting out a peak from the heart of the separation, it can be analyzed with higher separation efficiency on the second column. The run time of the second dimension analysis is usually longer than the collection time from the first dimension.
2D-LC 101
Explaining 2D-LC
On-line 2D-LC can be divided into “heart-cutting” and comprehensive approaches (see sidebar, “2D-LC 101”). Heart-cutting 2D-LC resolves components within a selected retention time window, while in comprehensive 2D-LC (my focus here) the entire sample is subjected to two separations. Actually, the first example of comprehensive chromatography was 70 years ago, in the separation of amino acids by paper chromatography (PC) (7): after developing in one direction with solvent A, the paper strip was turned 90 degrees and developed for a second time with solvent B. Given the static nature of the PC procedure, this was straight-forward.
In comprehensive 2D-LC, two columns are connected in series via a switching valve (modulator) and small-volume fractions from the first column effluent are collected and injected on the second column in multiple repeated alternating cycles. A standard comprehensive 2D-LC modulator is a ten-port switching valve with two collecting loops. Introduction of a successive fraction (loop 2) onto the secondary column can only be done when the previous fraction (loop one) elutes completely from the column. No restrictions are made on the analysis time on the first column but the analysis time (including regeneration time after the gradient) on the second column should be equal to or smaller than the modulation period. In an ideal comprehensive 2D-LC combination, the total peak capacity is that of the first column multiplied by that of the second column: nct = nc1 × nc2. Theoretically, this means that by coupling a column of nc1 500 (high resolution) with one of nc2 20 (high speed), nct is 10,000. However, the experimental nct is lower than the theoretical one as the 2D space can never be fully covered. Note that in comprehensive 2D-LC, the total analysis time is only slightly higher (typically 1 min) than the analysis time on the first column.
Optimum occupancy of the 2D space occurs when the separation mechanisms of the two dimensions have distinct retention profiles. The higher the orthogonality, the closer we get to theoretical peak capacity. Examples of high orthogonality include combining normal phase LC (NPLC) with reversed phase LC (RPLC); hydrophilic interaction liquid chromatography (HILIC) with RPLC; size exclusion chromatography (SEC) with RPLC; ion exchange chromatography (IEC) with RPLC; and even supercritical fluid chromatography (SFC) with RPLC. Surprisingly, although an RPLC × RPLC combination is, by definition, of low orthogonality, it provides interesting options for certain applications, such as peptide analysis at different pHs in the two dimensions (8). Moreover, in RPLC × RPLC, solvent incompatibility is not an issue and very robust methods, applicable in a QA/QC environment, can be developed as illustrated in Figure 1
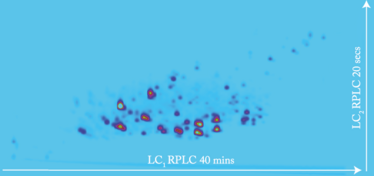
Figure 1. RPLC x RPLC 2D plot of polyphenols in green citrus tea recorded by UV at 320 nm. The first column is a C18 with a mobile phase gradient from 0.1% formic acid in water to methanol. The second column is a Phenyl-Hexyl with a mobile phase gradient from 0.1% formic acid in water to acetonitrile.
Comprehensive 2D-LC is mature enough to be very generally applicable, even in a routine environment; this is especially true with the use of robust instrumentation that offers both comprehensive and heart-cutting modes.
Pat Sandra and Gerd Vanhoenacker are at the Research Institute for Chromatography, Kortrijk, Belgium.
Further reading
“Comprehensive Chromatography in Combination with Mass Spectrometry”, Editor L. Mondello, (John Wiley & Sons Hoboken , NJ, USA, 2011).Agilent Technologies 2D-LC
Agilent Technologies Application Notes: tas.txp.to/0314/2D-LC
References
- J. C. Giddings, “Maximum Number of Components Resolvable by Gel Filtration and Other Elution Chromatographic Methods”, Anal. Chem. 39, 1027-1028 (1967).
- C. G. Horvath and S.R. Lipsky, “Peak Capacity in Chromatography”, Anal. Chem. 39, 1893 (1967).
- U. D. Neue, “Theory of peak capacity in gradient elution”, J. Chromatogr. A 1079, 153-161 (2005).
- G. Vanhoenacker et al., Agilent Technologies, Application Note 5990-4031 EN, 2009.
- J. M. Davis and J. C. Giddings, “Statistical Theory of Component Overlap in Multicomponent Chromatograms”, Anal. Chem. 55, 418-424 (1983).
- J. C. Giddings, “Sample Dimensionality: A Predictor of Order-Disorder in Component Peak Distribution in Multidimensional Separation”, J. Chromatogra. A 703, 3-15 (1995).
- R. Consden, A. H. Gordon, and A. J. P. Martin, “Qualitative Analysis of Proteins: A Partition Chromatographic Method Using Paper”, Biochem. J. 38, 224-232 (1944).
- I. François et al., “Tryptic Digest Analysis by Comprehensive Reversed Phase × Two Reversed Phase Liquid Chromatography (RP-LC×2RP-LC) at Different pHs”, J. Sep. Sci. 32 1137-1144 (2009).
Pat Sandra is Emeritus Professor of Organic Chemistry at Ghent University, and Founder and President of the Research Institute for Chromatography (RIC), Kortrijk, Belgium. “Through the activities of RIC, I got in touch with the real analytical needs of the industry and found we could help in providing solutions that are economically relevant. Moreover, it allowed me to keep my best PhD students around me, which resulted in high scientific output in a non-academic environment,” he says.