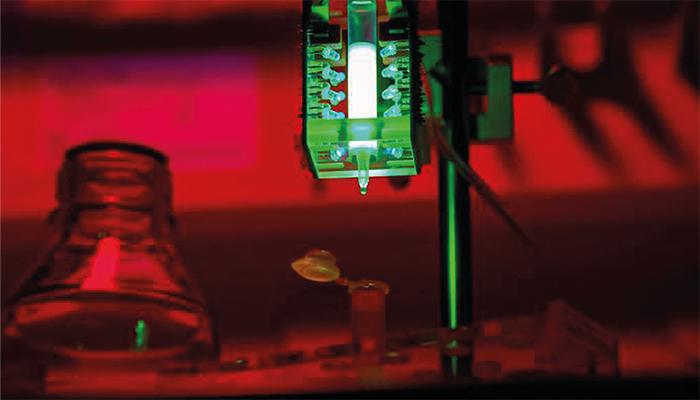
The team has further developed the method of chromatography. For this purpose, they have developed a light-sensitive molecular appendix that serves as an anchor for the target protein. It binds the target protein to a carrier material in the column. When the column is irradiated with mild UV light, the molecular appendix releases the carrier material and can be washed out with the target protein. For this purpose, the researchers have attached LED lights around the column. To visualize the process, the team is working here with a fluorescent protein.
Credit: Sabrina Bauer / TUM
A team at the Technical University of Munich (TUM) has developed a photochemical protein purification method that avoids the harsh elution steps of traditional affinity chromatography. By integrating a genetically encoded light-sensitive tag into target proteins, the researchers achieved reversible protein binding and release using mild ultraviolet light – without requiring chemical reagents.
The technique centres on a short peptide tag containing a non-canonical amino acid, azobenzene-lysine (AzoK – or “Azo-Tag”). This residue undergoes reversible cis–trans isomerisation when exposed to UV light at 355 nm. In its trans form, AzoK increases hydrophobicity and facilitates strong binding to hydrophobic interaction chromatography (HIC) resins; in the cis form, induced by UV irradiation, it becomes more polar and detaches from the resin.
“In contrast to conventional methods, we use a physical mechanism instead of chemical reagents,” said senior author Arne Skerra in a press release. “Our technology is fundamentally different, being both more gentle and more efficient.”
To test the system, the team expressed Azo-tagged variants of several proteins, including green fluorescent protein (GFP), an anti-HER2 single-chain variable fragment (scFv), and dihydrofolate reductase (DHFR), in E. coli. These proteins were purified using Butyl FF Sepharose resin packed into a column surrounded by UV LEDs. After binding in the dark, the tagged proteins were released in under 20 minutes upon irradiation, with recovery yields up to 90 percent and purities exceeding 95 percent.
Conventional affinity chromatography relies on chemical elution with acids or organic solvents, which can impair protein function. Here, the mild photorelease conditions preserved protein structure and activity, confirmed by CD spectroscopy and functional assays. The anti-HER2 scFv retained target binding after photopurification, as demonstrated by flow cytometry and immunofluorescence.
“This is a broadly applicable, non-denaturing approach to isolate proteins under physiological conditions,” the authors write.
The method also offers clear advantages in scalability. The chromatography column used in the study had a diameter of less than 1 cm, but the team anticipates that the system could be adapted for larger-scale purification in industrial settings. A patent has been filed, and work is ongoing to automate the process for high-throughput applications.
“We are currently working on automating the processes to make them even more efficient, especially for high-throughput drug development in pharmaceutical or biotechnology companies,” Skerra said.




