Though I hold the food science chair at JLU Giessen, I consider myself “free” from a research perspective, working regularly in pharmaceutical and environmental analysis.. I believe this freedom puts me in an excellent position to share advances in thin-layer chromatography (TLC) that have opened up new vistas in analytical capability. I was quite fortunate that I started my planar chromatography journey early under the supervision of “the pope” of quantitative high-performance TLC (HPTLC) – Helmut Jork at Saarland University, Saarbrücken. I was fond of HPTLC even then, but wanted to move into industry and, subsequently joined CAMAG – a leading HPTLC instrumentation company – for three years. It was some years later, during my time at the University of Hohenheim in Stuttgart in the group of Wolfgang Schwack (food chemistry), that I started working on planar chromatography and its hyphenation with mass spectrometry (MS). Today, every analytical scientist needs MS – it’s the universally accepted detector. I was frustrated by the lack of developments in planar chromatography – and I was not alone. Coupling TLC with mass spectrometry was absolutely essential in gaining as much information from the zone on the plate as possible. Why should we not replicate the crystal clear success seen in coupling MS with HPLC and GC?
I’ve always loved the visual nature of TLC, which offers the unusual ability to run parallel separations and compare and contrast directly. Moreover, you can answer questions very rapidly. But seeing the samples separated in front of me made me even more aware of the missing link: MS. Coupling such a useful and fast separation technique with mass spectrometry became both my passion and my ultimate goal. Ambient mass spectrometry was being explored at around the same time, and I could see synergistic benefits emerging between such ambient techniques and planar chromatography. On the elution head-based side of things, a colleague of mine – Heinrich Luftmann (University of Münster) – had attracted my attention with his paper, but the reliability was not proven and the technique was not yet convincing, especially as it only worked on flexible aluminum foils. However, I saw great potential. I visited his lab and explained that his approach made excellent sense; that it was an extremely practical solution to the problem; and that I wanted to develop it further. He had two prototypes of the ChromeXtractor and promptly gave me the older one. I started work and published a paper that described three important modifications that allowed coupling to glass plates (1). Over the course of three years, I published about 13 research papers on the optimization and application of the elution head-based interface, which was finally commercialized. Not satisfied with one coupling, I also published a paper on direct analysis in real time (DART) mass spectrometry (2). The DART paper won a “Highly Cited Author Award,” clearly showing how interested the community was in HPTLC-MS. Indeed, HPTLC – and TLC – had rather suddenly been dragged into the 21st century, and the profile of the technique was raised considerably.
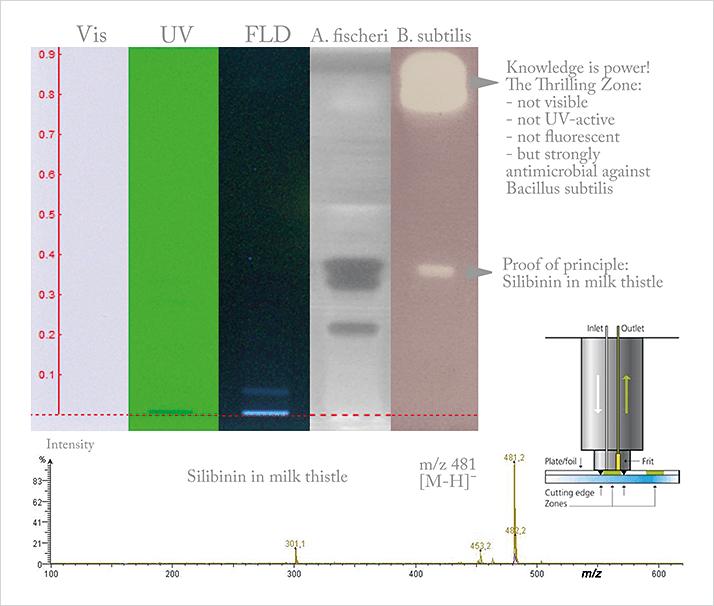
Coupling of (HP)TLC to mass spectrometry opened up a new world of applications. After those papers, I received many requests from companies who were interested in impurity analysis, particularly with respect to breakdown products in the pharmaceutical field. Previously in such situations, a particular zone would be scraped off the plate and then subjected to mass spectrometry – a laborious and error prone process. And in food analysis, the same benefits applied to contaminants or simple analyte confirmation. But the addition of mass spectrometry also made effect-directed analysis with HPTLC a reality. By using enzymatic assays or bioassays directly on the plate, we can quickly identify the zones of bioactive interest that can be further analyzed with mass spectrometry. For example, the cosmetics industry is very interested in plant extracts, and they want to understand more about bioactivity – the addition of mass spectrometry is essential in identifying the compounds of interest – and the use of HPTLC simplifies the process. In this magazine, multidimensional chromatography is a focus – complex separation techniques for complex samples and complex problems. And it sure is thrilling to get 4000 peaks from a sample. But sometimes that’s not enough – there may still be coelution – so where do we stop? Or perhaps we can identify 300 compounds – but what about the other 3700? Are they important? The use of HPTLC offers greatly simplified separation. It’s certainly no match for HPLC or GC in terms of resolution and separation number – but it can separate a complex mixture with little to no sample preparation. For sure, there is coelution – but when we are interested in bioactivity, the addition of a bioassay could reduce 4000 peaks in a complex sample to five bioactive zones for mass spectrometric analysis. It’s a beautifully streamlined approach that can give rapid answers to complex problems. And to address zone coelution, we can, for example in the case of a normal phase HPTLC, integrate a short C18 monolithic column between the plate and the MS, thus offering orthogonal separation – the time to result is just the same. In short, there is always more than one way to solve a problem.
Given its utility, why aren’t we all using advanced TLC-based approaches? Well, the reality is that the problem starts very early – in education. I’ve surveyed the problem for years, and in my experience the worldwide mean average teaching time for TLC is three minutes. A quick cursory introduction, a quick manual application to a plate, followed by development and the education is finished. It’s no surprise that the field has been very slow to develop. Consider HPLC and GC – teaching absolutely requires instrumentation (unlike TLC) and students assume that those techniques are more quantitative, more reliable or even more profound. I advise my teaching colleagues to buy HPTLC instrumentation, but they say, “We have limited budget. I can teach the principles with no instrumentation.” But that’s discrimination from the outset. In Germany we say “a tree must be bent while it is young”; the backwards-facing equivalent is “you can’t teach an old dog new tricks”... What does this mean for HPTLC? Well, we simply don’t have enough people working on HPTLC theory despite the potential.
So, you’re not a fan of sample preparation or the potential bias it introduces? Take a raw extract of your complex sample (feed or food, traditional medicine, supplement, nutraceutical, ...), subject it to HPTLC, couple it to a bio or enzymatic assay, and just see what happens. After all, HPTLC, especially when using area application on HPTLC plates, copes well with high matrix loads – we don’t reuse plates – and it can essentially do sample preparation and separation in the same step. And don’t forget, we see everything in thin-layer chromatography . If something is fixed at the starting zone, we don’t miss it, we just know we need to develop the same plate with a solvent mixture of a higher elution power. If something gets stuck in a HPLC column or a GC liner, you may never know – it never hits the detector. I worked with a huge pharmaceutical company that had spent half a year trying to figure out the difference between a good and bad batch using both HPLC and GC – they could not find differing peaks between the two samples. They came to me as a last resort and within half a day, we discovered that the difference was in the start zone. We re-analyzed the plate with a new solvent mixture and solved the problem. Unfortunately, the lack of education and training means that many people who finally try HPTLC may fail and say, “What a stupid method!”. But I assure you, it could well be an essential, highly complementary modern technique alongside those complex multidimensional chromatography methods already sitting in your toolbox.
References
- A. Alpmann and G. Morlock, “Improved Online Coupling of Planar Chromatography with ESI-MS: Extraction of Zones from Glass Plates”, Anal. Bioanal. Chem. 386, 1543-1551 (2006). G. Morlock and Y. Ueda, “New Coupling of Planar Chromatography with Direct Analysis in Real Time Mass Spectrometry”, J. Chromatogr. A 1143 (2007). M. T. Taha, M. B. Krawinkel, and G. E. Morlock, “HPTLC linked with (Bio)assays and Mass Spectrometry - a Suited Method for Discovery and Quantification of Bioactive Components?”, J. Chromatogr. A In Press (2015).




