Figure 1: Molecular structure of an Immunoglobulin
Introduction
Therapeutic recombinant antibodies, primarily of the Immunoglobulin G isotype (IgG) (figure 1), represent a growing proportion of biopharmaceuticals. There is an ever increasing number of approved antibody therapies available to treat a variety of modern-day diseases such as cancer, rheumatoid arthritis and diabetes. With many more candidate IgG's in the biopharmaceutical development pipeline, the pressure to accurately understand and develop these candidates becomes more and more important. Protein aggregation has long been established as a major concern in the biopharmaceutical industry. Proteins have a limited shelf-life and precipitate over time, with strong evidence that the presence of protein aggregates in the bloodstream can regularly stimulate the active/adaptive immune response. Size-exclusion chromatography (SEC) is an established and powerful tool that is commonly implemented to assess the aggregation content of protein formulations. SEC separates proteins by size, and is frequently employed to measure molecular weight and identify aggregate sample proportions. While most SEC systems use a single detector (such as an ultraviolet absorbance detector), the addition of a light scattering detector allows direct measurement of protein molecular weights and is used to differentiate between monomer, dimer and aggregate independent of retention volume. Light scattering is substantially more sensitive to aggregate formation than absorbance measurements and allows detection of aggregates that may otherwise be missed. If the light scattering detector employed is a multi angle light scattering (MALS), then it can also be used to measure the radius of gyration (Rg) of the large aggregates that scatter light anisotropically (unevenly with respect to angle), which offers further structural/shape information. The Viscotek SEC-MALS 20 system (figure 2) is a 20 angle light scattering device, capable of making measurements of protein and protein aggregate molecular weight, independent of elution volume.
Figure 2: The Viscotek SEC-MALS 20 instrument
Materials and methods
The SEC-MALS 20 detector was connected to a Viscotek TDAmax system using the TDA RI detector to measure concentration. Samples were separated along 2 x Viscotek protein columns. The mobile phase was phosphate buffered saline and the IgG sample was prepared in the mobile phase. The detectors and columns were all held at 30°C to ensure good separation and to maximize baseline stability.
Results
Figure 3 demonstrates that the IgG sample was successfully characterized using the Viscotek SEC-MALS 20. The data from the different MALS angles is shown in figure 4. The calculated molecular weights are shown in table 1. Table 1: Measured molecular weights of the different peaks in the IgG sample.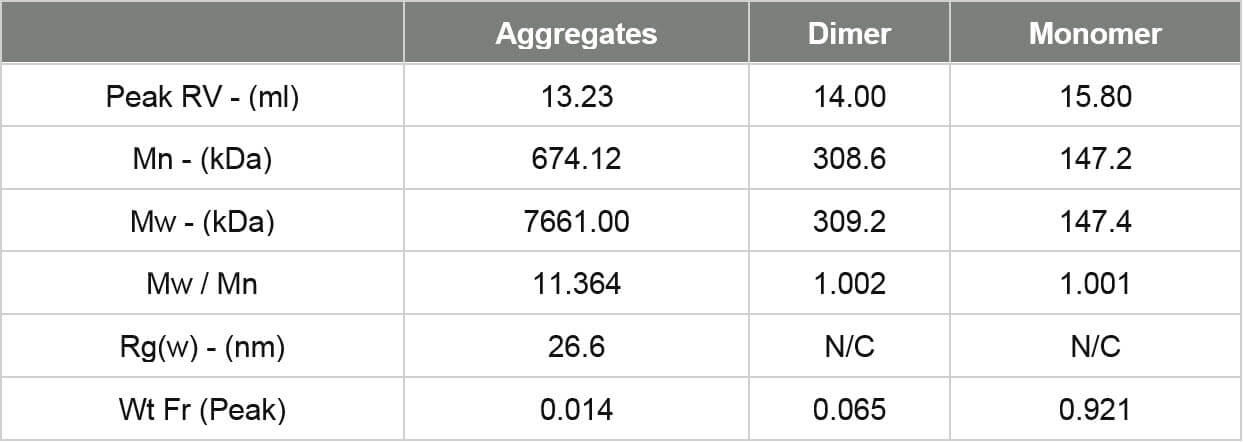
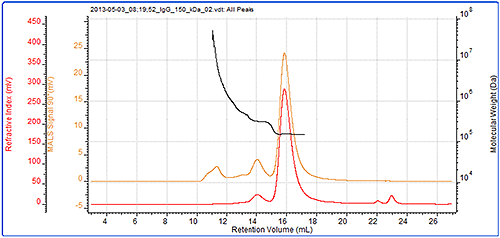
Figure 3: Chromatogram of IgG showing the refractive index (red) and MALS (90°) (orange) detector signals. The molecular weight measured by MALS is overlaid in black The results show that this sample of purified IgG is actually quite complex and has been separated into three peaks. The primary peak has a measured molecular weight of 147 kDa. This is very close to the IgG molecular weight and clearly identifies this sample as the monomer of IgG. The secondary peak has a measured molecular weight of just over 300 kDa and clearly identifies it as a dimer. The molecular weight across both of these peaks is very stable indicating that they are monodisperse. This suggests that the monomeric and dimeric forms of the IgG studied here are well-defined in terms of molecular weight and structure. The third peak, however, is quite different. Its light scattering signal is far larger than the refractive index signal and it has a correspondingly high molecular weight. The molecular weight is also highly variable, ranging from approximately 600 kDa up to 7x104 kDa. This peak is very polydisperse, as shown by the polydispersity value (Mw/Mn), indicating that the range of molecular weights within this peak is very broad. This is suggestive of non-specific aggregation. It is likely that any IgG molecules in these aggregates will have lost most or all of their activity.
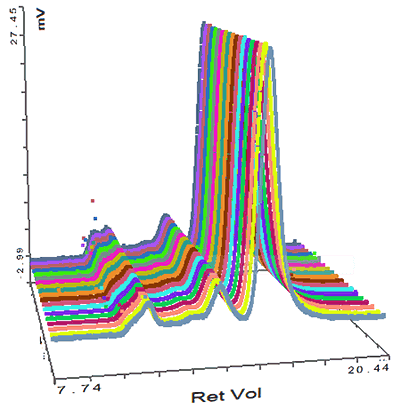
Figure 4: Overlay of MALS detector responses for IgG The concentration detector allows the individual populations to be quantified; their proportions are also shown in the table. The monomer peak comprises 92% of the sample while the dimer represents just 7% of the sample. The large aggregates make up less than 2% of the sample. Proteins are relatively small molecules by the standards of SEC and MALS. Soluble proteins are typically less than 10 nm by radius and are isotropic scatterers, which means their Rg cannot be measured using MALS. This is true for the monomeric and dimeric forms of IgG observed here. Some of the largest aggregates, however, are just large enough to be anisotropic scatterers and for their Rg to be measured. Studying figure 4, it is just possible to see some angular dependence in the scattering of the aggregate peak. Analyzing this gives an average Rg of 26.6 nm. Figure 5 shows a corresponding Zimm plot taken from the top of the aggregate peak. A Zimm plot from the top of the monomer peak, shown in figure 6, clearly demonstrates that the IgG monomer is an isotropic scatterer.
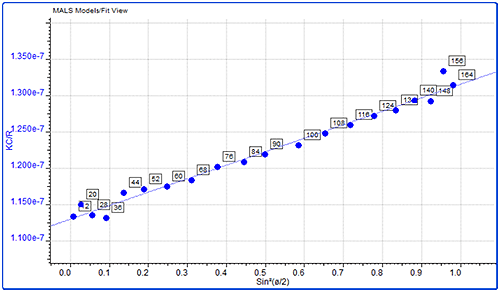
Figure 5: Zimm plot showing the anisotropic scattering for the aggregate peak
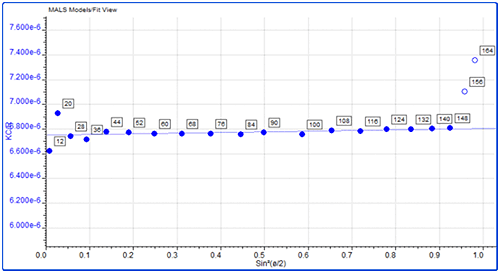
Figure 6: Zimm plot showing isotropic scattering for the monomer peak
Discussion
This application note has described the successful measurement of the molecular weight of IgG using the Viscotek SEC-MALS system. This instrument can measure protein molecular weight independent of a protein's elution volume or structure, and molecular size in the form of Rg for molecules large enough to display anisotropic scattering. The molecular weights of IgG monomer and dimer were successfully measured and the molecular weight and Rg of the IgG aggregates were also characterized. The SEC-MALS 20 is an perfect additional detector for any SEC instrument to provide a system with a high quality MALS capability, giving direct measurement of protein molecular weights.Malvern provides the materials and biophysical characterization technology and expertise that enables scientists and engineers to investigate, understand and control the properties of dispersed systems. These systems range from proteins and polymers in solution, particle and nanoparticle suspensions and emulsions, through to sprays and aerosols, industrial bulk powders and high concentration slurries. Used at all stages of research, development and manufacturing, Malvern’s instruments provide critical information that helps accelerate research and product development, enhance and maintain product quality and optimize process efficiency. Our products reflect Malvern’s drive to exploit the latest technological innovations. They are used by both industry and academia, in sectors ranging from pharmaceuticals and biopharmaceuticals to bulk chemicals, cement, plastics and polymers, energy and the environment. Malvern systems are used to measure particle size, particle shape, zeta potential, protein charge, molecular weight, mass, size and conformation, rheological properties and for chemical identification, advancing the understanding of dispersed systems across many different industries and applications. www.malvern.com Material relationships http://www.malvern.com/en/ portal@malvern.com






