“During my graduate studies in the early 80’s I had the chance to work with GC and HPLC instruments from Hewlett-Packard. When we received our first 1090 HPLC, I was so impressed that I wanted to work with the people that had developed this system,” says Monika Dittmann. She got that opportunity in 1988 and has worked for the company (now Agilent Technologies) ever since, developing instruments and technologies in HPLC, capillary electrophoresis, capillary electro-chromatography and microfluidics.“I like to work in a multi-disciplinary environment,” Dittmann says, “only by combining expertise from different areas can we able to meet the evolving needs of our customers.”


Utilizing the full power range of high-performance liquid chromatography (HPLC) instruments is constrained by constant flow operation. How can we improve separation speed and robustness of current ultra high-performance liquid chromatography (UHPLC) separation methods by removing these shackles?
When we consider the basic thermodynamic principles of liquid chromatography (LC), the retention factor of an analyte is governed by the volume ratio of stationary and mobile phases and a peak elutes after a certain volume of mobile phase has passed the column, independent on flow rate (1). The original definition of the retention factor k’ in LC is given by:
k’ = K • Vs / Vm
where:K = distribution coefficient of the solute
Vm = volume of mobile phase
Vs = volume of stationary phase The elution (retention) volume is given by
Vr = Vm • (k’+1)
In the early days of LC, the driving force separating ions was gravity and the identification of compounds was achieved by elution volume and spectral properties. With the advent of modern HPLC it became common practice to record an elution time rather than elution volume. This was mostly for a good, practical reason: it is easier to measure time than to measure elution volume. There is, however, a prerequisite for interchangeability of run volume and run time, which is operation at a constant flow rate. Only when this is preserved can the run volume be determined directly from the run time.Vrun(t) = ∫0 F(t)dt = F • t
What price is being paid to keep the flow rate constant during a chromatographic separation? We give up the flexibility to operate our system at the highest possible separation speed, since flow rate has to be adjusted to solvent properties such as maximum viscosity. In constant flow operation, flow rate is limited by the pressure drop at the composition with maximum viscosity and it is not possible to speed up the separation when the viscosity decreases. To illustrate this, imagine running a gradient separation using water and an organic solvent like acetonitrile or methanol. The solvent properties, such as viscosity, heat capacity, and density vary with the composition of the eluent. As a result, the pressure drop and temperature gradient due to frictional heating vary across the gradient. For a gradient of acetonitrile and water the viscosity reaches a maximum (1.2 cp at 20°C) at 20% acetonitrile and decreases to a minimum (0.45 cp @ 20°C) for pure acetonitrile. This means that large parts of the gradient could be run at higher flow rates if we were free to vary the flow over the course of a run (see Figure 1).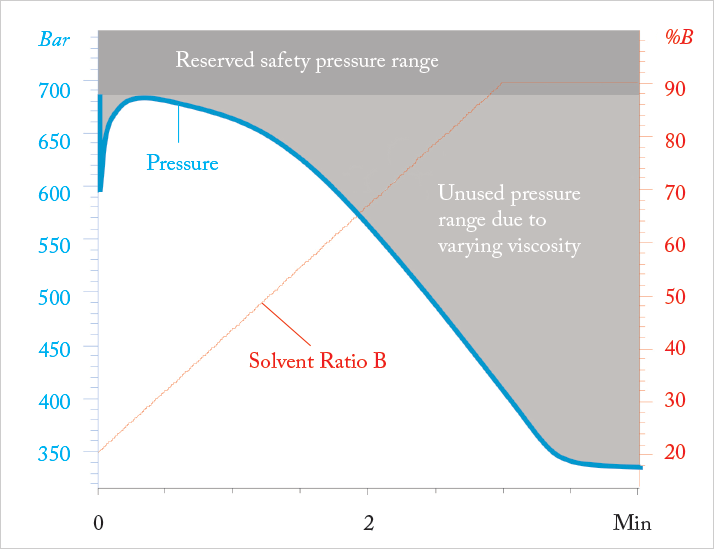
In UHPLC separations, the power generated by the pump leads to a temperature increase along the length and the radius of the column. The magnitude of this increase depends on pressure drop, heat capacity, density and adiabatic expansion factor of the eluent. As all these values vary during a solvent gradient, the column excess temperature varies significantly over a run. When the temperature equilibration times are long (10 – 30 min) compared the gradient time, this can lead to differences in temperature profile in a series of gradients. With variable flow operation, the flow rate could be adjusted to keep the excess column temperature constant, leading to better retention time reproducibility.
If the constraint of constant flow rate is removed from HPLC operation, different operation modes become possible. This opens up new opportunities for control and optimization, such as operation at constant pressure, operation at constant frictional heat, and real time flow adjustment for special applications. These applications could include stopped-flow operation for HPLC- nuclear magnetic resonance (NMR) coupling or fractionation, selectively increasing or decreasing flow at specific time points in a separation. In pressure-controlled operation, the flow rate would be adjusted to keep the pressure drop constant, being low in the high viscosity region and increasing with decreasing viscosity. This has a number of potential benefits: the overall separation time would be decreased, there would be no shutdowns due to overpressure and the pressure stress on columns would be reduced (2-5). In frictional heating-controlled operation, the variation in temperature increases over a series of gradient runs could be reduced, leading to improved reproducibility of retention times and resolution (6, 7). What is needed to enable volume-based HPLC? The following are prerequisites:
- The gradient shape, that is, composition versus run volume, must be maintained independently of flow rate variations. To provide clearly defined and reproducible gradients, programs must be based on run volume rather than on elapsed time.
- Accurate, real-time data on run volume at varying flow rate must be available for evaluation of detector signals versus run volume.
- Software tools must be available that use the run volume data to rescale the signal versus real time into signal versus run volume data as these are independent of the speed with which the peak passes the detector.
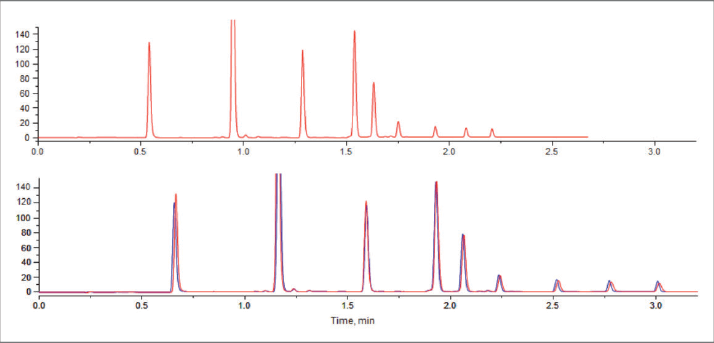
To achieve these goals, the pump must be capable of measuring the run volume in real time and adjusting the solvent composition such that the delivered gradient in volume units remains as programmed, independent of flow rate variations. Such a pump requires a controller with increased computing power and very precise control of piston position to make the necessary changes to the volume displaced in real time. However, the value of primary importance for volume-based operation is the decompressed solvent volume after the column outlet. The Agilent 1290 series pumps can provide this information reliably given the very low system elasticity and the availability of accurate compressibility tables for the solvents used, which allow exact calculation of the decompression ratio. Precise knowledge of the run volume is needed to (a) control the gradient formation in volumetric units in real time and (b) to rescale the detector signal with respect to run volume or virtual time (that is, the time that would have elapsed if the separation had been run at a selected constant flow rate). It is important to note that the gradient is programmed by the operator in time units for a certain flow rate, just as in conventional HPLC operation. The gradient is then executed by the pump in volumetric units exactly as programmed, independently of the actual flow rate used. The chromatogram and analysis of data can be performed with respect to run volume or in virtual time, depending on the preference of the user. Figure 2 shows chromatograms obtained at constant pressure (1000 bar, upper) and at constant flow (0.6 ml/min, lower). In the lower trace, the constant pressure chromatogram is converted to virtual time and overlaid. The time savings that can be obtained in constant pressure operation will depend on the type of organic modifier and the start and end composition of the gradient. In practice, time gains of up to 25 percent can be anticipated, taking into account that the pressure safety margin can be reduced.
Volume-based LC could be used in virtually all areas of modern liquid chromatography. The increased separation speeds would have great impact on high-throughput applications or in speeding up comprehensive 2D-LC. Another potential application area is routine HPLC, where the volume-based operation mode could be used to maximize robustness by avoiding shut-downs due to overpressure. The operation of columns at constant pressure might also provide greatly enhanced column lifetime, although this needs further evaluation.
References
- L.R. Snyder, “Linear elution adsorption chromatography: VII. gradient elution theory”, J. Chromatogr., 13, 415-434 (1964). K. Broeckhoven, et al., “Kinetic performance limits of constant pressure versus constant flow rate gradient elution separations. Part I: Theory”, J. Chromatogr., 1218, 1153-1169 (2011). M. Verstraeten, et al., “Kinetic performance limits of constant pressure versus constant flow rate gradient elution separations. Part II: Experimental”, J. Chromatogr., 1218, 1170-1184 (2011). M. Verstraeten, et al., “Comparison of the quantitative performance of constant pressure versus constant flow rate gradient elution separations using concentration-sensitive detectors”, J. Chromatogr., 1232, 65-76 (2012). M. Verstraeten, et al., “Quantification aspects of constant pressure (ultra) high pressure liquid chromatography using mass-sensitive detectors with a nebulizing interface”, J. Chromatogr., 1274, 118-128 (2013). F. Gritti and G. Guiochon, “Gradient chromatography under constant frictional heat: Realization and application”, J. Chromatogr., 1289, 1-12 (2013). F. Gritti and G. Guiochon, “Realization and potential advantages of gradient separations performed under steady state temperature regime”, J. Chromatogr. 1291, 104-113, (2013).




