
A recent study from researchers in Finland and the UK has challenged the belief that heart attacks are triggered only by cholesterol and lifestyle factors. Using molecular microbiological techniques, including real-time quantitative PCR and immunohistochemistry, the team presents evidence that infectious processes – detectable at the DNA and tissue level – may also be involved in the onset and progression of heart disease. We connected with lead researcher Pekka J. Karhunen to learn more about how these bacterial signals were identified in coronary plaques, the analytical challenges of working with calcified vascular tissue, and what the findings could mean for future diagnostic strategies and myocardial infarction prevention.
What inspired this study?
Acquired risk factors such as elevated cholesterol, hypertension, smoking, obesity, and physical inactivity account for roughly 75 percent of the excess risk of coronary heart disease (CHD). However, nearly half of patients with cardiovascular disease present with few or no traditional risk factors, and up to 20 percent of those who experience myocardial infarction lack any classical risk factors. Genome-wide association studies have largely reinforced the link between coronary artery disease and lipid- and inflammation-related pathways, but – despite early expectations – the strongest genetic variants explain only about 10 to 15 percent of CHD variability when considered alongside known risk factors. This gap suggests that additional, unidentified contributors may drive disease onset and progression.
The idea that infectious agents might contribute to chronic inflammation within coronary plaques has existed for decades, but the theory was widely dismissed after large antibiotic trials failed to demonstrate benefit. Interest has resurfaced with the advent of molecular microbiological methods capable of detecting bacterial DNA in very small quantities within biological specimens.
Our renewed curiosity in this question arose somewhat by chance: while testing a new bacterial DNA detection technique on coronary autopsy samples, we were surprised to find DNA from several oral bacteria within the plaques. Initially, we suspected contamination and struggled to publish the finding. Meanwhile, we observed that middle-aged victims of sudden cardiac death had more dental infections than individuals who died from other causes. Taken together, these observations laid the foundation for the project that ultimately culminated in our publication.
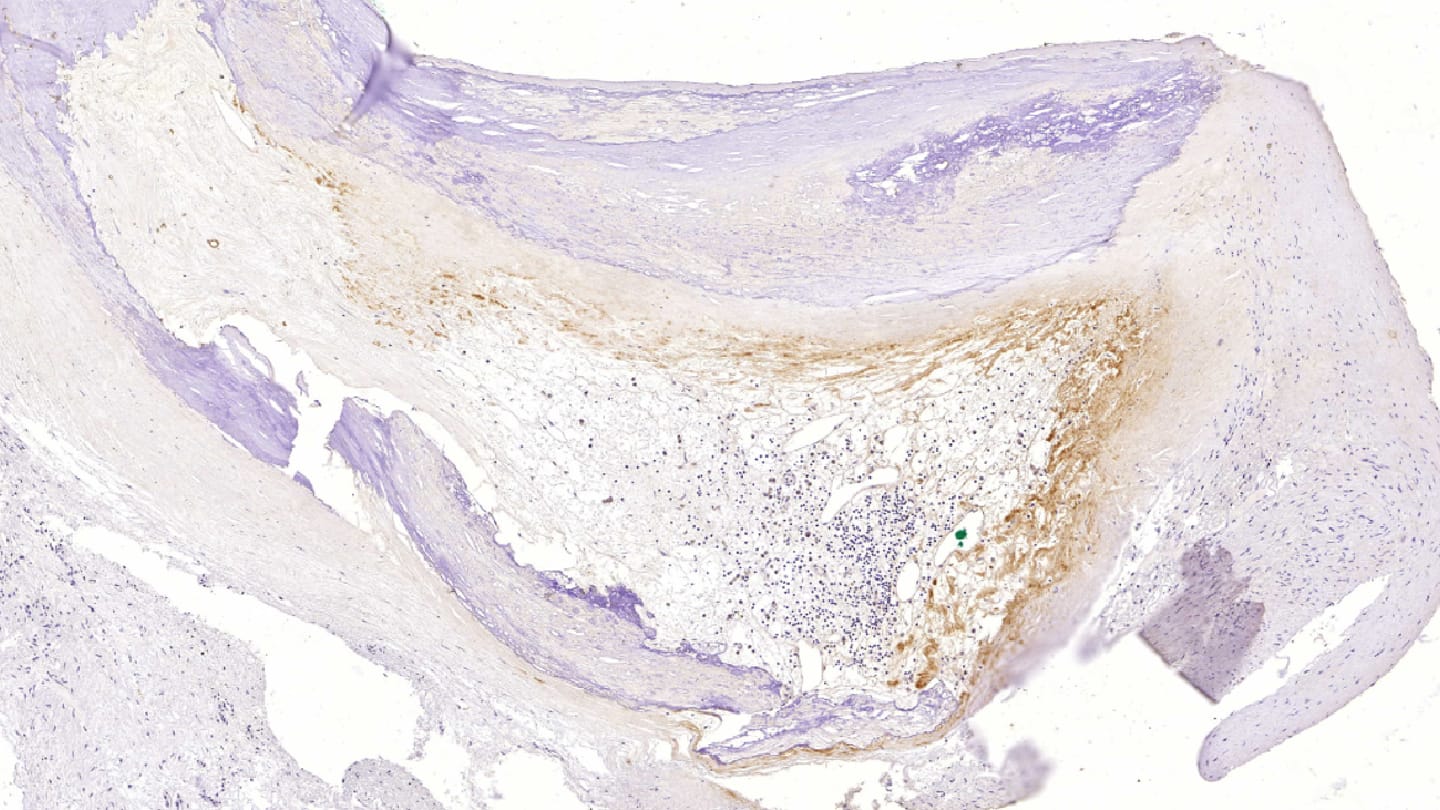
Did you face any challenges during your research, and if so, how did you overcome them?
The main challenge, of course, was that our initial material consisted of postmortem coronary samples, raising the possibility that the detected bacteria were contaminants. However, contaminant organisms do not trigger immune recognition or an inflammatory response – both of which we were able to demonstrate in these samples. In addition, we analyzed carotid, aortic, and femoral artery specimens from surgical patients and replicated both the bacterial DNA findings and the immunohistochemical staining results. This consistency across living and postmortem material further argues against postmortem contamination.
We also encountered significant technical difficulties when preparing the heavily calcified arterial samples for histology. Sectioning them on a microtome and ensuring the sections adhered to slides proved challenging. The samples required up to two weeks of EDTA decalcification, and EDTA had to be applied directly to the block immediately before cutting to obtain sections with as little fragmentation as possible. It also took time to identify microscope slides capable of holding these sections effectively. Extracting DNA from calcified tissue was similarly demanding and required extensive optimization.
Could the methods you used be adapted for use in diagnostic or hospital labs?
At this stage, these methods are used primarily in basic translational research. Currently, there are no commercial antibodies available for viridans streptococci (with the exception of Streptococcus pneumoniae), as these organisms are generally regarded as part of the normal oral flora.
How might your findings change how clinicians interpret inflammation in atherosclerotic plaques?
It has long been assumed that the inflammation and inflammatory cells within atherosclerotic plaques arise from an autoimmune response to oxidized LDL, which was thought to be the primary driver of inflammation. Emerging evidence now suggests that oral bacteria may also contribute to this process. These organisms appear to persist as a dormant, symptomless biofilm within the plaque for years and may trigger inflammation when they reactivate. Their presence is likely enabled by neovessels that develop in response to hypoxia within the plaque, providing a route for bacterial entry.
What are the next steps for this research?
In hopes of improving outcomes for patients with myocardial infarction, we will begin an antibiotics trial next year. The plan is to administer a short, three-day course of antibiotics immediately after the infarction diagnosis is made – at the point when virulent bacteria are most likely present within the ruptured atheroma. Within just a few days, these bacteria may be phagocytized by macrophages, leaving behind a biofilm that is largely resistant to antibiotic therapy. This timing issue likely explains why previous large, long-term antibiotic trials were unsuccessful, as treatment often began only after significant delays, sometimes months after the acute event.
We are also investigating whether calcification of atherosclerotic plaques may be related to calcification of bacterial biofilms within the atheroma. Our unpublished data suggest that the same oral bacteria responsible for forming dental biofilm – which later calcifies into dental calculus – may also form a calcified biofilm inside the plaque.




