Legal Highs & Lows
As new psychoactive substances (NPS) flood the market and “designer drug” sales are on the rise, we are faced with significant – and growing – social and analytical challenges. Here, I offer an overview of a quietly unraveling crisis.
Amira Guirguis |
While serving as a hospital pharmacist, I frequently encountered patients with marijuana hidden in their belongings; often, they declared them as personal “herbal medicines”. At the same time, the use of “designer drugs” was increasing and it was not uncommon to encounter numerous admissions with unknown sources of toxicities. The lack of available clinical guidelines for treating designer drug-related toxicity made it challenging and led to the provision of supportive adjunct treatment as well as treating the symptoms themselves. Furthermore, symptom control was based on local formularies used by different hospitals rather than national guidelines (1). These challenges inspired me to undertake a PhD research project to study and investigate the new flood of “designer drugs”. This has led me into contributing to two EU-funded projects that focus on the problem of NPS: EU-MADNESS (www.eumadness.eu) and Enhancing Police Skills regarding NPS (www.npsproject.eu).
No control = crisis
NPS are synthetic drugs made for recreational use that are not subject to international control (2). To circumvent the law, NPS manufacturers slightly modify the structure of established drugs of abuse, while retaining their pharmacological effects (3) (Table 1). NPS are sold and marketed with labels that do not reflect the actual content of the product. Indeed, they are often branded with confusing and frankly ridiculous names such as “Dr. Booga Shooga” or “meow meow”, labeled as “research chemicals”, “legal highs”, “food supplements”, “bath salts”, “plant food”, or “herbal highs”, with complex acronyms, such as AH-7921 or 251-NBOMe (or Nbomb). The ban of one drug has been shown to be associated with rapid replacement by an alternative “legal and uncontrolled” drug and has actually resulted in the proliferation of the NPS market, with increased diversity of products, users, distributors and risks.
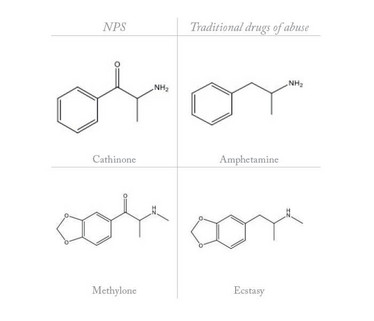
Table 1: Structural similarity between emerging NPS and traditional drugs of abuse
In contrast to the limited number of traditional drugs of abuse, such as cocaine, heroin and ecstasy, the number of NPS is increasing dramatically and there is no sign of the market slowing down. Internet sales and the ease with which new online markets are created contribute to the crisis. Currently, the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) is monitoring more than 500 NPS. The EMCDDA has also reported that more than two drugs appeared on the market each week in 2014. And the reported number of NPS seizures in Europe has also increased seven-fold between 2008 and 2013 (4).
The EMCDDA categorizes NPS as piperazines, benzodiazepines, arylamines, tryptamines, opioids, phenethylamines, synthetic cathinones, synthetic cannabinoids and others (see Figure 1). Synthetic cathinones and synthetic cannabinoids have been the most popular classes of NPS in Europe since 2009. According to the EMCDDA report (March 2015), the NPS market has expanded to include “legal highs” (marketed with bright packaging and attractive brands, and sold in headshops and over the Internet); “research chemicals” sold over the Internet “for scientific research” (with labels imitating safety data sheets). Such NPS are more attractive to “psychonauts”, who like to try “new stuff” rather than get addicted to one drug. NPS also include food supplements sold in fitness shops and over the Internet and “designer drugs” sold as traditional drugs of abuse by drug dealers in the illicit drug market. The products often contain one or more NPS but can also contain diverted prescription medicines sold by drug dealers in the illicit drug market.
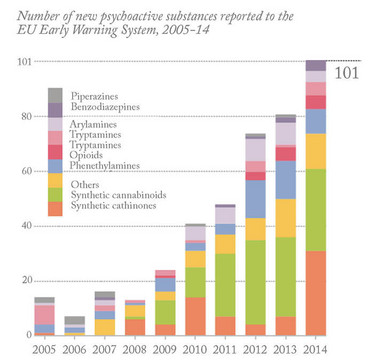
Figure 1: New psychoactive substances in Europe. An update from the EU Early Warning System (March 2015, Reproduced with permission from the EMCDDA)
The term “legal highs” is undesirable and misleading. It gives the impression that these products are legal and safe when they may contain controlled substances (drugs or their precursors) and harmful contaminants. It also gives the impression that these NPS induce “highs”, when in fact some of them are depressants or have overlapping pharmacological effects.
In addition, there is evidence that NPS are used in combination with traditional drugs of abuse and/or alcohol. In other cases, NPS replace the use of traditional drugs of abuse; for example, heroin injectors are now moving onto injecting mephedrone because it is cheaper but has similar psychoactive effects.
A recipe for social disaster
NPS emerged after the drug cookery books written by Alexander (Sasha) and Anna Shulgin became available in the late 1990s (5)(6). In 2009, NPS appeared in huge numbers and became internationally popular for different reasons primarily because they are legal, cheap, “undetectable”, and provide desired effects such as euphoria, increased sociability, elevated mood, hallucinations, increased libido and so on. Users of NPS include people of all ages and persuasions: students, members of the LGBT community, people interested in ‘chemsex’, heroin users, clubbers, gym-users, the homeless population, prisoners, and users of substance misuse and needle-and-syringe-exchange schemes. Knowledge of the patterns of NPS abuse mainly depends on anecdotal self-reported user surveys and user experiences shared in drug fora, for example Erowid and Bluelight. NPS can be snorted, smoked, injected, ingested (sometimes via bombing, in which the drug is placed in paper, rolled into a ball and ingested) or dissolved in alcohol.
Toxic ignorance
Information regarding NPS intoxication is very limited due to the lack of data from hospital emergency departments, walk-in centers, and so on. Nevertheless, the EMCDDA issued 16 public health alerts in 2014 for NPS associated with serious harm, such as hospitalisation and death. Published case studies highlight some of the toxic effects of NPS and include UK cases of sympathomimetic toxicity from the intake of mephedrone (8), attempted murder resulting from the combined intake of 3-methoxyphencyclidine (3-MeO-PCP) and methylenedioxypyrovalerone (MDPV) (9), lethal serotonin syndrome after the combined ingestion of methylone and butylone (10), and death from taking the diet pill, 2,4-dinitrophenol (or DNP) (11). NPS effects on different body systems such as the sympathetic nervous system, cardiovascular, or neurological systems is dependent on the amount taken, and the number and type of drugs co-ingested/injected, all of which have different clinical implications.
The 2011/12 Crime Survey for England and Wales (CSEW) has reported that ketamine users generally have high rates of simultaneous poly-use (12). Ketamine has also been linked to significant sexual health risks (13) and the prevalence of premature death among the drug-injecting population (14). For mephedrone alone, a synthetic cathinone, presentations for treatment rose in England from 839 in 2010/11 to 900 in 2011/12 amongst clients aged 18 years and over (15). And the number of mephedrone-related TOXBASE accesses (a primary clinical toxicology database) was 7061 in 2013/14, 8432 in 2011/12 and 6169 in 2011/12 (16–18).
The explosive emergence of NPS, the anonymity of Internet sales and the emergence of “cryptomarkets” are posing great challenges for policy-makers and law-enforcement agencies. NPS are not currently under any international control, although many countries have established permanent control measures for some NPS or issued temporary bans. For example, in April 2010 the UK classified cathinones as controlled drugs under Schedule II of the Misuse of Drugs Act 1971 through a generic definition i.e., banning all emerging NPS that are made by slightly modifying the generic chemical structure of cathinones. Conversely, selected cathinones were placed under temporary orders in the USA such as the cathinone 4-MEC (4-methylethcathinone), which was only classified as a Schedule I controlled drug in 2014. The downside of the generic definition ban of classes of NPS is that some new analogs have fallen outside of the generic definition and became legal analogs to controlled drugs. Naphyrone, which emerged to the market following the ban in July 2010, is an example. Therefore, the generic definition was modified to include it. This was followed by the emergence of bk-2C-B, which remains a legal cathinone in the UK.
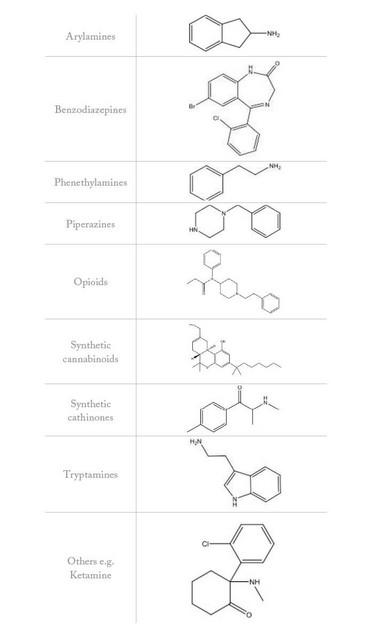
Figure 2: Example chemical structures for NPS categories (EMCDDA, 2015)
Root of the problem
India and China produce large quantities of legal high products. And the fact that they can also be manufactured from legal precursors of known tested pharmaceuticals facilitates large-scale production. The drugs are exported legally to Europe, where they are cut and distributed across the vast Internet market. The drugs are typically cut with a variety of controlled or uncontrolled active ingredients, prescription medicines and inert substances. Figure 2 shows example chemical structures for NPS categorised by EMCDDA.
Limited information is available on the pharmacology and toxicology of NPS (19)(20). NPS may exhibit stimulant, depressant, empathogenic, aphrodisiac, dissociative, hallucinogenic, entactogenic and psychotropic effects. Most NPS exhibit psychoactive and sympathomimetic features (21), with the possibility of NPS classes overlapping and sharing one or more psychoactive effects (22) because most street drugs are sold as racemic mixtures.
Potencies, toxicities, pharmacokinetics and pharmacodynamics depend on whether the drug exists as a single enantiomer or a mixture of both enantiomers. If both enantiomers exist, their dynamics may be altered due to potential synergistic or competitive actions. Additionally, the enantiomeric fraction may change over time because of preferential metabolism of one over the other (23). Let’s take just one example: cathinone analogues exhibit various pharmacological effects, which include stimulant, empathogenic and anti-depressant effects (24)(25). Coppola et al (2012) and Cozzi et al (1999) showed that cathinones might exert their stimulant effect by inhibiting the enzymes tyrosine hydroxylase and tryptophan hydroxylase, which are responsible for the synthesis of dopamine (DA) and serotonin (5-HT) (26)(27). Cathinones also inhibit the re-uptake of the neurotransmitters DA, 5-HT and norepinephrine (NE) by the monoamine transporters, which then reduces clearance of neurotransmitters from the synaptic cleft. Additionally, they induce the release of newly synthesised monoamines from the cytoplasm as well as stored monoamines from the synaptic vesicle stores. These pharmacological effects result in reduced concentrations of monoamines in the frontal cortex, hippocampus and neostriatum. Reduced catecholamine concentrations have been shown to extend for up to 30 days leading to the destruction of monoaminergic neurons (28)(29).
Specialized, portable, in-field analysis
The global proliferation of NPS is posing international public health risks and a pronounced burden for first responders. In-field detection of NPS is crucial for law enforcement agents, where the identification of unknown compounds is important for making decisions (for example, making an arrest). Fortunately over the past decade, handheld techniques have become available, which have the advantage of bringing the lab to the sample (7). A recent development includes the use of surface-enhanced Raman spectroscopy (SERS – for more, see "Gurus of Raman Spectroscopy") for the detection of mephedrone, with a limit of detection of 1.6 µg mL-1 (30). Presumptive tests have also been used for the identification of cathinones (31), but because such tests depend on the presence of a functional group, they can lead to false positives. Other rapid tests include immunoassays, which are commonly used for in-field detection of drugs of abuse (32) and NPS in biological matrices, such as urine (33). These kits are limited by the fact that they must be developed for known specific drugs and cannot be used for unknown, new NPS (33). In addition, their excellent selectivity prevents the identification of NPS analogues due to low cross-reactivity (34), which may yield false negative results (32)(35). An on-the-spot screening instrument was recently developed and involved the use of disposable electro-analytical sensors for identifying three cathinones (36). Yet, the latter studies investigated NPS samples in solution rather than in solid state.
Handheld techniques employing Fourier transform infrared or Raman spectroscopy are the main analytical techniques currently employed by forensic scientists for the screening and identification of NPS (see “War on NPS” for more). Gas chromatography-mass spectrometry (GC-MS) is considered the main “wet” chemical technique used in forensic labs for the analysis of seized NPS or NPS and their metabolites in body fluids such as urine. So, should a sample test positive in the field, it may be transported to a forensic lab and tested using GC-MS. High performance liquid chromatography (HPLC) is also used for quantification of selected NPS.
Oliver Sutcliffe (Senior Lecturer in Psychopharmaceutical Chemistry at MMU) holds an electrochemical NPS sensor. (Photo courtesy of Manchester Metropolitan University)
We need you!
Certainly, analytical chemistry plays an important role in screening, identifying and quantifying NPS products; rapid identification and quantification ensures swift arrests/confiscations, aids in treatment decisions in healthcare settings, and assists with preventing the widespread distribution of harmful substances. However, we still need more analytical developments to overcome the challenges of “designer drugs”. For example, we lack reference standards for many drug samples because of the pace at which new drugs emerge on the market and the cost of synthesizing them. To evade detection and circumvent the law, clandestine chemists have been able to produce heterogeneous NPS products (37). And as mentioned earlier, NPS are often intentionally branded and mislabeled, which adds complexity and makes the identification of NPS and the discrimination between NPS and excipients difficult. Other challenges faced include the presence of contaminants, coloured powders and unknown constituents and limitations of conventional in-field immunoassay kits. For example, signals resulting from excipients intentionally mask and hinder NPS identification. Finally, NPS products may contain controlled drugs, which requires analytical labs to hold specific expensive licenses, and thus hinders “designer drug” research.
Traditional harm reduction techniques are difficult to apply to NPS because they are continuously emerging with fantasy names, different mixtures, novel analogues, precursors and diverse chemical structures. It is crucial to develop evidence-based harm reduction services by raising awareness and educating the public. Exchange information between different countries through the projects like the European Early Warning System is also indispensable. Despite being debatable, new policy is essential when it comes to tackling the NPS problem; the coming into force in April of the UK’s Psychoactive Substances Act 2016 is aimed at effecting a “blanket ban” on NPS without hindering NPS-related research, but it may simply lead to increased clandestine activities.
So far, clandestine chemists have always been one step ahead. In addition to developing more efficient on-site testing to avoid false positives and false negatives, analytical chemists need to collaborate with forensic and law enforcement agencies and service providers to predict future generations of NPS. We need methods that unambiguously identify these drugs before they can cause harm.
- L Motto, “Emergency Room Response to Bath Salts”, (2012). laurenmotto.yolasite.com/resources/motto_U2D2.pdf
- “The challenge of new psychoactive substances, in the Global SMART Programme, United Nations Office on Drugs and Crimes, Vienna, Austria (2013).
- LD Simmler et al., “Pharmacological characterization of designer cathinones in vitro”, Br J Pharmacol, 168(2), 458-471 (2013).
- M Evans-Brown., “New psychoactive substances in Europe. An update from the EU Early warning System”. European Monitoring Centre for Drugs and Drug Addiction (EMCDDA), Luxembourg (2015).
- A Shulgin and A Shulgin,“Pihkal: A chemical love story”, (1995), Transform Press, ISBN: 0963009605, 9780963009609
- A Shulgin and A Shulgin,“Tihkal: The continuation”, (1997), Transform Press, ISBN: 0963009699, 9780963009692
- S Assi et al., “Analysis of ‘legal high’ substances and common adulterants using handheld spectroscopic techniques”, Anal Methods, 7, 736-746 (2015)
- DM Wood et al., “Recreational use of mephedrone (4-methylmethcathinone, 4-MMC) with associated sympathomimetic toxicity”, J Med Toxicol, 6(3), 327–30 (2010). PMID: 20358417.
- R Stevenson and L Tuddenham, “Novel psychoactive substance intoxication resulting in attempted murder”, J Forensic Leg Med, 25, 60–1 (2014). PMID: 24931864.
- BJ Warrick et al., “Lethal serotonin syndrome after methylone and butylone ingestion”, J Med Toxicol, 8(1), 65–8 (2012). PMID: 22160789.
- S Morris, “Woman died after accidental overdose of highly toxic diet pills”, (2015). www.theguardian.com/society/2015/jul/23/woman-died-accidental-overdose-highly-toxic-diet-pills-eloise-parry.
- “Crime survey England and Wales 2011”, The Home Office, London, UK (2012).
- L Mitcheson et al., “Sexual health risk among dance drug users: cross-sectional comparisons with nationally representative data”, Int J Drug Policy, 19(4), 304–10 (2008). PMID: 18638703.
- “UNODC, world drug report”, United Nations Office on Drugs and Crimes: Vienna, Austria (2015).
- “Club drugs: emerging trends and risks”, National Treatment Agency for Substance Misuse: London, UK (2012).
- “NPIS, National Poisons Information Service – Annual Report 2011/2012”, Health Protection Agency: London, UK (2012).
- “NPIS, National Poisons Information Service – Annual Report 2012/2013”, Health Protection Agency: London, UK (2013).
- “NPIS, National Poisons Information Service – Annual Report 2013/2014”, Health Protection Agency: London, UK (2014).
- A Green et al., “The preclinical pharmacology of mephedrone; not just MDMA by another name”, Br J Pharmacol, 171(9), 2251–68 (2014). PMID: 24654568.
- MJ Valente et al., “Khat and synthetic cathinones: a review”, Arch Toxicol, 88(1), 15–45 (2014). PMID: 24317389.
- ME Nelson et al., “Emerging drugs of abuse”, Emerg Med Clin North Am, 32(1), 1–28 (2014). PMID: 24275167.
- D Zuba and B Byrska, “Prevalence and co‐existence of active components of ‘legal highs’”, Drug Test Anal, 5(6), 420–9 (2013). PMID: 22549997.
- S Evans et al., “Chiral illicit drugs and pharmaceuticals–stereoselective degradation, and its implications for analysis, toxicology and regulatory frameworks”, Joint Annual Meeting of the Ecotoxicology Research and Innovation Centre Plymouth University, and the Society of Environmental Toxicology and Chemistry UK Branch, University of Bath, UK (2013).
- “Consideration of the cathinones”, Advisory Council on the Misuse of Drugs: London, UK (2010).
- Erowid (2013). www.erowid.org/psychoactives/psychoactives_def.shtml.
- M Coppola and R Mondola, “Synthetic cathinones: chemistry, pharmacology and toxicology of a new class of designer drugs of abuse marketed as “bath salts” or “plant food””, Toxicol Lett, 211(2), 144–9 (2012). PMID: 22459606.
- NV Cozzi et al., “Inhibition of plasma membrane monoamine transporters by β-ketoamphetamines”, Eur J Pharmacol, 381(1), 63–9 (1999). PMID: 10528135.
- M Sparago et al., “Neurotoxic and pharmacologic studies on enantiomers of the N-methylated analog of cathinone (methcathinone): a new drug of abuse”, J Pharmacol Exp Ther, 279(2), 1043–52 (1996). PMID: 8930215.
- MP Gygi et al., “Role of endogenous dopamine in the neurochemical deficits induced by methcathinone”, J Pharmacol Exp Ther, 283(3), 1350–5 (1997). PMID: 9400010.
- S Mabbott et al., “Optimization of parameters for the quantitative surface-enhanced Raman scattering detection of mephedrone using a fractional factorial design and a portable Raman spectrometer”, Anal Chem, 85(2), 923–31 (2013). PMID: 23198960.
- “The recommended methods for the identification, and analysis of amphetamine, methamphetamine and their ring-substituted analogues in seized materials”, United Nations Office on Drugs and Crimes: Vienna, Austria (2006).
- T Biermann et al., “On-site testing of illicit drugs: the use of the drug-testing device “Toxiquick””, Forensic Sci Int, 143(1), 21–5 (2004). PMID: 15177627.
- SD Brandt et al., “The new drug phenomenon”, Drug Test Anal, 6(7–8), 587–97 (2014). PMID: 24995418.
- KN Ellefsen, et al., “Validation of the only commercially available immunoassay for synthetic cathinones in urine: Randox drugs of abuse v biochip array technology”, Drug Test Anal, 6(7–8), 728–38 (2014). PMID: 24659527.
- S Kerrigan et al., “Evaluation of commercial enzyme-linked immunosorbent assays to identify psychedelic phenethylamines”, J Anal Toxicol, 35(7), 444–51 (2011). PMID: 21871153.
- 36. JP Smith et al., “Forensic electrochemistry: the electroanalytical sensing of synthetic cathinone-derivatives and their accompanying adulterants in “legal high” products”, Analyst, 139(2), 389–400 (2014). PMID: 24287637.
- TJ Weatherholt, “Not for human consumption: how inept legislative policy proliferates the synthetic drug problem”, Kentucky Law Journal, 103, 1–12 (2015).
Before training as a pharmacist, Amira Guirguis started out as an accountant, then she became an interpreter and translator. She is currently undertaking her PhD at the University of Hertfordshire focusing on developing spectral and computational models for the identification of novel psychoactive substances solid mixtures. Her previous research focussed on transdermal permeation of topical pharmaceuticals and the elemental profiling of herbal medicines. Amira is also an active member of the committee of the London North West Local Pharmacy Forums (LNWLPF) a mentor at the Royal Pharmaceutical Society (RPS) and a pre-registration pharmacist trainer at Propharmace. She is an associate of the EU MADNESS project and a member of the General Pharmaceutical Council (GPhC), the Royal Pharmaceutical Society (RPS) and the Royal Society of Chemistry.