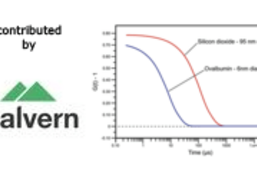
Latest articles in Techniques & Tools
Latest articles in Fields & Applications
Latest articles in Business & Education
Latest Product Profiles
Latest Application Notes

Read more
The Power List 2023
The Analytical Scientist Power List returns to celebrate the successes of the field’s leading lights!
Read more
Read more
The Power List 2022
Read more
The Power List 2021
Around the World in 60 Scientists
The Power List 2020
All Power Lists