NASA’s Curiosity Rover (Figure 1 (a)) recently raised its head (camera) to the night sky to take its first photograph of Earth from the surface of the Red Planet (Figure 1 (b)). The picture postcard shot strongly evokes the words of Carl Sagan:
“From this distant vantage point, the Earth might not seem of any particular interest. But for us, it's different. Consider again that dot. That's here. That's home. That’s us. On it everyone you love, everyone you know, everyone you ever heard of, every human being who ever was, lived out their lives.”
– Pale Blue Dot: A Vision of the Human Future in Space.

Figure 1. a) Artist’s impression of the Curiosity Rover.

Figure 1. b) Earth – the brightest spot in the Martian twilight.
But, apart from snapping photos of Earth and taking “selfies,” has Curiosity provided any analytical insight? Towards the end of 2013, Curiosity passed the milestone of 100,000 “zaps” of its ChemCam laser ablation breakdown spectroscopy (LIBS) instrument. The landmark infrared laser blast was fired from distance of 4.04 meters at a rock called “Ithaca” (naming rocks on Mars is a bit of a pastime for NASA – tas.txp.to/0214/rocks). Each pulse delivers more than one million watts of power for a tiny fraction of a second – roughly five one-billionths. The resulting plasma spark is observed by ChemCam’s “telescope” to gain spectroscopic information about rock composition (see Figure 1c). The international team pouring over the resulting data is interested in learning about the diversity of materials inside Mars’ Gale Crater and the geology involved in their formation.
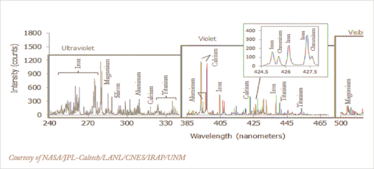
Figure 1 c) Spectrum recorded by ChemCam’s LIBS instrument. The spectrum averages data from multiple laser firings from the same rock sample spot and is typical of Martian volcanic material, and identifies a standard major-element suite of silicon, magnesium, aluminum, calcium, sodium, potassium, oxygen and titanium.
To learn more about LIBS, please see “Beginning to See the Light”.

Rich Whitworth completed his studies in medical biochemistry at the University of Leicester, UK, in 1998. To cut a long story short, he escaped to Tokyo to spend five years working for the largest English language publisher in Japan. "Carving out a career in the megalopolis that is Tokyo changed my outlook forever. When seeing life through such a kaleidoscopic lens, it's hard not to get truly caught up in the moment." On returning to the UK, after a few false starts with grey, corporate publishers, Rich was snapped up by Texere Publishing, where he spearheaded the editorial development of The Analytical Scientist. "I feel honored to be part of the close-knit team that forged The Analytical Scientist – we've created a very fresh and forward-thinking publication." Rich is now also Content Director of Texere Publishing, the company behind The Analytical Scientist.