Scalable Microfluidics Reveal Hidden Microbial Metabolite Interactions
A new fluidic system allows researchers to track microbial communication in real time – uncovering key metabolite exchanges in complex communities
| 2 min read | News
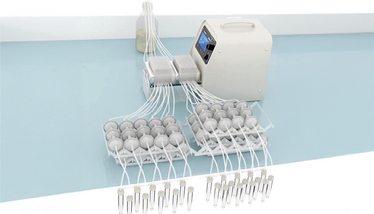
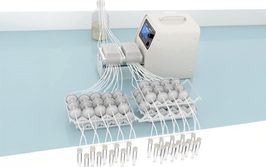
llustration depicting a MetaFlowTrain system with 24 metabolic lines, each containing 2 microchambers connected in series.
Credit: Stéphane Hacquard
Researchers at the Max Planck Institute for Plant Breeding Research have developed a scalable and modular fluidic system that enables dynamic, real-time investigation of metabolite-driven interactions between microbial species. The new system, MetaFlowTrain, overcomes previous limitations in studying microbial metabolic exchange by enabling continuous culture and compartmentalization of microbial communities.
Microbial communities communicate and compete via secreted exometabolites – small molecules such as amino acids, organic acids, and secondary metabolites. Identifying which species produce specific exometabolites – and how these affect others – has remained challenging, as conventional methods such as co-culturing or batch supernatant assays often conflate physical contact effects with chemical exchange and cannot capture time-resolved dynamics.
MetaFlowTrain houses microbes in isolated 3D-printed microchambers separated by 0.22 µm filters, which allow only metabolites to pass between chambers. These microchambers can be linked into “trains” with directional flow driven by a peristaltic pump, enabling structured experimental layouts. Each chamber allows non-destructive temporal sampling and supports microbial growth via continuous nutrient flow, reducing artefacts caused by nutrient depletion and metabolite accumulation common in static systems.
“The staggering diversity of molecules produced by microbes is a result of millions of years of evolution,” said Stéphane Hacquard, lead author of the study, in a recent press release. “These molecules serve different functions that help these communities to survive, adapt, and thrive in various environments.”
The researchers validated the system using bacterial and fungal strains from the Arabidopsis root microbiome and observed that even in nutrient-poor peat extracts, continuous flow sustained microbial proliferation. Metabolite profiling using both targeted and untargeted mass spectrometry showed distinct exometabolite signatures between strains, with 37.2 percent of variation in untargeted metabolite profiles and nearly 50 percent in targeted profiles attributable to strain identity. Further experiments demonstrated the system’s ability to detect unidirectional interactions. Exometabolites from bacterial communities strongly inhibited fungal growth – an effect absent when using a triple-mutant Pseudomonas strain lacking key antimicrobial exometabolites.
The team also used MetaFlowTrain to show how soil conditioning – such as previous exposure to Arabidopsis and microbial communities – altered downstream microbial assembly and metabolite output, with implications for plant health. Metabolites collected from microchambers influenced Arabidopsis germination rates and root growth in bioassays.
With flexibility to suit a range of analytical workflows – including qPCR, amplicon sequencing, and targeted or untargeted LC-MS – the authors hope their system has potential to identify natural antimicrobial compounds and study host-microbe-metabolite interactions under defined conditions. “Understanding the functions and the mode of actions of some of these molecules will drive agricultural innovations," Hacquard noted.