Smart Bandage Monitors Chronic Wounds in Real Time
iCares device enables non-invasive, continuous biochemical analysis of wound fluid in patients
| 3 min read | News
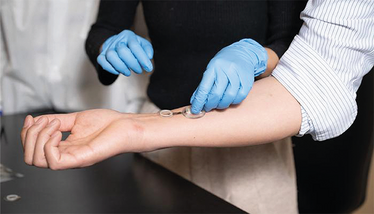
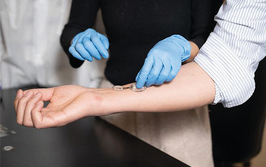
A smart bandage that promotes healing and monitors wound conditions is applied to the skin of a human volunteer.
Credit: Caltech
A wearable “lab-on-skin” device developed by researchers at Caltech and the Keck School of Medicine of USC has demonstrated continuous, real-time monitoring of chronic wounds in human patients, offering a potential tool for personalized wound care and early infection detection. The study details the clinical evaluation of the device – called iCares – on 20 individuals with non-healing diabetic or venous ulcers.
The iCares system combines a triad of passive microfluidic components with a nanoengineered electrochemical sensor array to detect a suite of biomarkers, including nitric oxide (NO), hydrogen peroxide (H₂O₂), oxygen, pH, and temperature. These markers provide insight into tissue perfusion, inflammation, and bacterial infection. "Our innovative microfluidics remove moisture from the wound, which helps with healing," said senior author Wei Gao in the press release. "They also make sure that samples analyzed by the bandage are fresh, not a mixture of old and new fluid."
The device was worn directly over wounds and captured exudate through a series of carefully engineered structures: a Janus membrane with asymmetric wettability, cactus-inspired wedge channels, and a micropillar array for directed fluid transport and refreshing. This design enabled pump-free fluid handling while maintaining high sample fidelity – critical for analyzing slowly oozing, low-volume wound fluid.
Electrochemical detection was achieved through miniaturized sensors printed on a flexible polymer substrate. The NO sensor, for example, used a laser-engraved graphene/graphene oxide electrode modified with hemoglobin, operating at a negative potential to avoid interference from other electroactive species in the wound matrix. Additional sensors quantified H₂O₂ using Prussian blue-modified electrodes, and oxygen using nanoporous gold. The system was integrated with a wireless circuit board for data acquisition and Bluetooth transmission to a user interface.
In mouse models, the iCares patch successfully detected elevated temperature, pH, and H₂O₂ prior to the appearance of visible infection symptoms, enabling early intervention. In human subjects, the device captured dynamic shifts in wound biochemistry over time and across clinical interventions, such as vascular surgery. “iCares can watch in real time for important biomarkers of inflammation and infection,” Gao said.
To interpret the multiplexed biochemical data, the team developed a machine learning pipeline capable of classifying wound types and predicting healing trajectories. Using features such as NO, H₂O₂, and patient factors like age and peripheral artery disease status, the model achieved classification accuracies of 88.8 percent for wound severity and 94.0 percent for healing time prediction. These performance levels approached the accuracy of expert clinical assessment.
The system’s components were manufactured using inkjet printing, laser cutting, and 3D printing – allowing for scalable, low-cost production. The disposable sensor array is paired with a reusable circuit module, keeping long-term use economically viable.
The authors envision future iterations integrating therapeutic components, such as drug delivery or oxygen supplementation, to enable not just monitoring but active wound management.