Breath Test for Silicosis Delivers Results in Minutes
William Alexander Donald, Professor of Chemistry at UNSW, details his team's new chromatography-free diagnostic workflow, which combines direct-injection APCI-MS and machine learning to analyze breath samples in less than two minutes
Henry Thomas | | 6 min read | Interview

William Alexander Donald
A team from UNSW Sydney has developed a new breath-based diagnostic workflow for silicosis, a serious and often irreversible lung disease caused by inhaling fine silica dust. The new method bypasses the need for chromatography by combining mass spectrometry with machine learning analysis, delivering rapid, non-invasive results in less than two minutes – a potential game-changer for occupational health screening programs.
To find out more about the team’s development story and how their method compares to more traditional approaches to health screening, we reached out to William Alexander Donald, Professor of Chemistry at UNSW and co-lead author of the study.
Could you explain, in a nutshell, how your diagnostic method for silicosis works?
Using a custom-built, low-volume atmospheric pressure chemical ionisation (APCI) source, we’ve developed a rapid diagnostic workflow to directly analyze breath samples without the need for chromatography. Subjects exhale into Tedlar bags at the clinic, followed by sample analysis via direct injection into a relatively compact benchtop ion trap mass spectrometer within six hours. Per sample, the mass spectrometry analysis takes less than two minutes.
We chose to use an ion trap in order to keep costs low and enable deployment in mobile vans, clinics, or large work sites like mines. Our goal is to capture a broad profile of volatile organic compounds in each breath sample, before applying our interpretable, lab-developed machine learning models to classify the breath profiles. As the test is both fast and non-invasive, it has the potential to screen anywhere from a dozen – up to over a hundred – workers per day.

Postgraduate student Laura Capasso, Prof. William Alexander Donald and study lead author Merryn Baker look at results of breath samples analysed by AI for early detection of silicosis in a UNSW Chemistry laboratory.
Credit: UNSW Sydney/Richard Freeman
What was the biggest analytical challenge you faced during development, and how did you overcome it?
One of the main challenges arose in optimizing the volume of the ionisation chamber. Initially, we made the chamber very small to boost sensitivity, which worked well and generated strong signals for trace-level volatile organic compounds. Over time, however, we noticed a slow signal drop-off – it's most likely this was due to a build-up of space charge, as the chamber volume was simply too small for stable operation. This kicked off a round of rapid prototyping, in which we tested a range of different materials and configurations. Eventually, we landed on a design that gave us the right balance – where the chamber was large enough to avoid charge accumulation, but compact enough to achieve high sensitivity. This represented a turning point, as it gave us the stability we needed to run multiple samples.
Another key step was in simplifying the ion source itself. We originally experimented with a nanosecond pulsed ion source from a previous project, which granted us more sensitivity, but was ultimately too finicky for routine use without better engineering. Switching to a more robust APCI design made the system far more consistent and easier to operate. Surprisingly, the data side came together quickly. We used interpretable machine learning scripts from earlier projects, and once we had enough samples, we obtained high AUC values almost immediately. Seeing the first AUC curve come through marked the “lightbulb moment” when we knew the approach had potential.
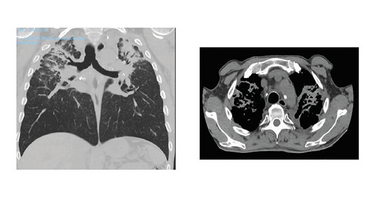
CT scans of a tunneller diagnosed with silicosis. The light grey areas at the top part of the lungs represent severe scar-tissue.
Credit: UNSW Sydney/Deborah Yates
How does your method compare to more conventional diagnostic techniques such as GC-MS?
Unlike gas chromatography–mass spectrometry (GC-MS), which typically requires lengthy preconcentration steps and long run times, our method is direct and fast. By eliminating chromatography and instead using a direct-injection APCI approach, samples are analyzed in under two minutes with no preconcentration. This method prioritizes speed and ease of use to enable rapid profiling of complex breath samples, and we’ve obtained sub-ppb detection limits for some test compounds without requiring sample preparation.
What are your thoughts on the wider potential of tools that combine mass spectrometry and machine learning?
The integration of mass spectrometry with interpretable or explainable machine learning provides the means to effectively extract diagnostic information from complex biological samples. Our group previously applied this approach to metabolomics data from blood plasma for early Parkinson’s disease detection, using an AI-based tool we developed called Classification and Ranking Analysis using Neural network generates Knowledge from Mass Spectrometry (CRANK-MS). The novelty lies in analyzing full spectral data with advanced machine learning methods like neural nets, which account for relationships between features that are easy to miss when considering each variable in isolation. These tools can be adapted for a range of diseases, and the ability to explain the model’s predictions is an important quality. As we continue with this research, we envision AI-powered MS becoming a cornerstone of rapid diagnostics.

Merryn Baker and Laura Capasso at a clinic in Sydney where engineered stone worker Angus Read provides a breath sample to be analysed for signs of silicosis.
Credit: UNSW Sydney/Richard Freeman
Could your method complement (or even replace) current methods used in occupational health surveillance programs?
Current screening relies heavily on chest X-rays, CT scans, and spirometry – tools that typically only detect silicosis after significant (and often irreversible) lung damage has occurred. Spirometry can be widely deployed and is commonly used in surveillance programs, but it lacks the sensitivity to detect early disease. CT scans provide more definitive results, but with a hefty cost and limited accessibility – especially in remote or high-volume settings. Our method offers a complementary approach that is rapid, non-invasive, and well-suited for frequent screening. With breath collection taking under a minute and complete mass spectrometric analysis in under two, the test could be used to screen anywhere between dozens and over a hundred workers per day – a feat considered impractical with CT imaging, the current “gold standard.”
Importantly, our AI model has a high negative predictive value – already above 90% – meaning it could be potentially deployed as a first-line triage tool. Workers flagged as low risk could avoid unnecessary follow-ups, while those flagged as higher risk could be directed for further clinical evaluation. This would help occupational health teams prioritize resources and potentially even detect diseases before symptoms emerge.
What are the next steps in translating your research to a more scalable tool?
The next step is large-scale, real-world validation. We’ve already established a second testing site in the Hunter Valley, close to the location of thousands of at-risk workers, to explore the use of breath testing in screening. Alongside this, we’re refining our machine learning models with larger datasets, which will improve predictive performance and robustness across diverse populations. To further scale the technology, we aim to move from broad profiling toward a more targeted panel format, identifying key discriminatory features that could eventually support regulatory approval.
Ultimately, our goal is to create a relatively compact, user-friendly, and affordable diagnostic platform that can be used routinely to bring the early detection of silicosis into everyday occupational health practice.
William Alexander Donald is Professor in the School of Chemistry, UNSW Sydney
Deputy Editor of The Analytical Scientist

















