The Analysis of Residual Solvents in Pharmaceutical Products Using GC-VUV and Static Headspace
Introducing a new method for faster sample throughput and shorter GC runtimes
sponsored by VUV Analytics
Volatile organic compounds are used in pharmaceutical manufacturing during the production of drug substances, pharmaceutical additives, and drug products. Known also as residual solvents, they account for 50-90 percent of mass in typical pharmaceutical operations and represent most of the process toxicity. Testing for the presence of these solvents, therefore, is critical for patient safety.
Testing commonly follows United States Pharmacopeia (USP) Method <467> guidelines, which suggest a gas chromatography (GC) runtime of 60 min. In addition, it is recommended that Class 1 and 2 solvents be identified and quantitated by complementary methods utilizing different stationary phase selectivity when solvents are found to meet or exceed the permitted daily exposure (PDE) concentration limits.
In this unique application, a gas chromatography–vacuum ultraviolet (GC-VUV; VUV Analytics VGA-100) method was developed to significantly reduce the analytical bottleneck involved in the testing of residual solvents. Experiments were undertaken to demonstrate the chromatographic capabilities of GC-VUV when applied to residual solvent analysis using model pharmaceutical matrices such as generic throat spray. Samples were prepared by mixing 2-mL of water with 25-100 mg/mL of pharmaceutical product and spiking Class 2 Solvent Mix A and B (Restek) to meet the desired concentration limits. The total GC runtime was set to eight minutes and used throughout the GC-VUV residual solvent application.
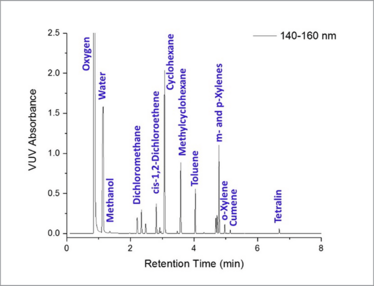
Figure 1. Compressed gas chromatogram showing Class 2 residual solvents separated and identified in less than eight minutes. The typical runtime for the USP 467 method is 60 min.