Electrifying Analysis in R&D
Electrochemical reaction cells are finding new applications in the pharma R&D lab that could offer big time and cost savings. Here, we share our experiences using electrochemistry with on-line mass spectrometry to study pharmaceutical stability and oxidation products – and explain why we’re electrochemistry converts.
Mark R. Taylor, Susana Da Silva Torres |
Electrochemical reaction cells have been used in analytical chemistry for over 50 years (1). Exploiting nature’s redox reactions of organic species, these systems have enabled us to achieve selective and sensitive detection in chromatography (2) as well as benchmarking stability of products susceptible to oxidative degradation (3). However, until recently, the use of electrochemistry as a method of producing redox products for further study has not been routinely applied in the majority of pharmaceutical research and development laboratories.
The pioneering work of Professor Uwe Karst and his co-workers at the University of Münster has shown that commercial lab-scale electrochemical reaction cells (in our case a Roxy Potentiostat system from Antec, the Netherlands) can be routinely applied to generate pharmaceutical metabolite profiles very similar to those observed in vivo and in vitro using enzymatic digestion (4). There are obvious advantages to being able to produce metabolites of a drug substance quickly and cleanly in real-time using electrochemistry with on-line mass spectrometry (EC-MS) rather than traditional in vitro methods using expensive xenobiotic metabolizing enzymes or cell digestions, where lengthy sample preparation for MS is often required due to potential interference from the sample matrix. Use of a reaction cell with on-line MS or LC-MS system allows for much more rapid and convenient study of redox metabolites and the EC-MS technique is starting to be applied more widely as a result, giving faster access to key data on metabolite profiles and structure. The oxidized species produced in these reactions are often unstable; by studying them in real-time, we can avoid bias from product decomposition during sample preparation and storage.
Stability testing
As well as in vivo metabolism, oxidation plays a major role in pharmaceutical stability and, along with hydrolysis, is one of the most common mechanisms of drug degradation. Pharmaceutical companies go to great lengths to understand and control these potential degradation mechanisms in their products. Gaining an understanding of the theoretical and real oxidation product profile of each new pharmaceutical product is a regulatory expectation and of fundamental importance to protecting patient safety and ensuring robust and relevant stability indicating methods, to provide a basis for stability studies. Extensive stress testing through forced degradation is routinely applied using a range of in silico and in vitro methods designed to cover all possible routes of potential degradation including thermal, humidity, photo and chemical (hydrolytic and oxidative) stress tests (5).
Just like the metabolic studies we mentioned earlier, electrochemical reaction cells coupled to MS and LC-MS give us a new and convenient way of studying the redox stability of pharmaceutical products. We can now study reactions on-line and in real-time using high-resolution accurate mass MS, which is able to churn out proposed chemical formulae of products in a matter of seconds (6). The use of electrochemical cells obviates the need for lengthy and often hard-to-replicate chemical treatments using caustic reagents, such as hydrogen peroxide. By fine-tuning the applied cell potential we can optimize the process to achieve the maximum yield of specific target reaction products prior to on-line or off-line analysis by complementary spectroscopic techniques, such as nuclear magnetic resonance (NMR) or bioassay (7).
An addictive example
We used EC-MS in direct infusion mode to study naltrexone, a potent narcotic antagonist used in maintenance treatment for opiate and alcohol dependence, which is known to degrade by oxidation (9). Figure 1 shows an overlaid ion intensity plot of a naltrexone standard solution, prepared in a simple ammonium acetate electrolyte buffer, as it is syringe-pumped through an electrochemical reaction cell into a high-resolution mass spectrometer. As the applied cell potential was linearly increased, the intensity of the naltrexone substrate mass ion (m/z = 342.1705) decreased and ion intensities from naltrexone oxidation products increased and decayed as new oxidation products are formed. Mass ions consistent with formation of a dimer (2-2’-bis-naltrexone), dehydrogenated naltrexone and associated dimer (M-2H) and from the addition of one, two and three oxygen atoms (M+16, M+32, M+48) to naltrexone were observed as the oxidation potential was increased. It was interesting to observe the relationship of the different products to applied voltage – you can see the formation and decay of reactive intermediates and products in real-time as voltage was increased. Analysis of the redox cell effluent by LC-MS suggests that isomers of the oxygenated reaction products are formed from different sites of hydroxylation. The results were consistent with what we already know about the drug – species formed at lower potentials are observed in laboratory stability studies and special stabilization agents have been proposed to mitigate against their formation in pharmaceutical products (8). The ability to gather data by EC-MS without the need for specialist reagents and reaction time-course sampling is hugely attractive and gives us a head start to stability-relevant data.
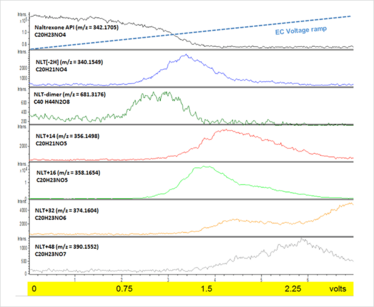
Figure 1. EC-MS ion intensity plots of infused naltrexone and electrochemical cell oxidation products using a magic diamond electrode and applied voltage ramp (0-3V in 7 minutes).
Amplifying oxidation products
Once pharmaceutical oxidation products cross a particular concentration threshold relative to the active pharmaceutical ingredient (API), regulators expect their structures to be identified. Markers of the oxidation products are often needed to validate stability indicating methods and to ascertain their relative response factors in the selected quantitative analytical methods. It can be quite a challenge to identify trace (≤ 0.1 percent) levels of oxidation products, as they can easily be drowned out by the relatively large quantities of main-band API. In our laboratories, we have used EC-MS and, more recently, preparative synthetic electrochemistry to accelerate identification of the oxidation products. By electrochemically depleting the amount of API relative to that of the oxidation product it is quite possible (by selecting the optimum cell potential) to increase the concentration of an observed trace oxidation product to 50 percent or more of the total chromatogram peak area in just a few minutes (Figure 2). This provides enough material for more sophisticated LC-MS-MS experiments to be performed and for structure confirmation and concentration measurement using NMR, without having to resort to complex sample preparation techniques.
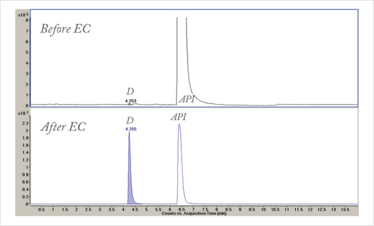
Figure 2. Overlaid LC-MS chromatograms of a pharmaceutical API sample showing enrichment of a trace degradant (D) following electrochemical oxidation (EC).
Making oxidation markers
Synthesizing API oxidation product markers for method development and validation can be technically challenging using traditional wet chemistry approaches, often requiring weeks of chemist time, plus sourcing and evaluation of starting materials and reagents. We wanted to see if we could use an electrochemical synthesis cell to produce oxidation product markers rapidly and directly from solutions of the API dissolved in electrolyte (9). Using fesoterodine as a model compound, we were able to produce the oxidation products directly from the API without the need to source special reagents (Figure 3). Better yet, we observed that the reaction was much more rapid and selective than in-house attempts to produce these oxidation products using traditional approaches. Attempts to produce larger amounts of material (starting with 80 mg substrate) were successful too, with the trade-off that the total conversion rate in two hours was reduced to approximately 75 percent. A mixture of products was produced so individual pure products were recovered using preparative HPLC and centrifugal evaporation.
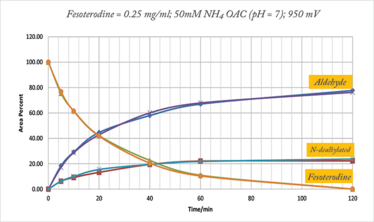
Figure 3. Overlaid curves showing the rate of formation of two target oxidation products produced by electrolysis of 20 mg fesoterodine fumarate (9).
As early adopters, we approached the use of EC-MS to support pharmaceutical stability and structure elucidation studies with trepidation. We were concerned that the technology would fail to deliver in a high-pressured and busy laboratory. However, in every case, electrochemistry has provided a time and labor cost saving that has easily repaid the capital investment. We are now using the technique routinely to facilitate understanding of pharmaceutical oxidative stability, enable structure elucidation and simplify synthesis of oxidation product markers, and we expect to see more and more laboratories joining us in the years ahead.
Mark R. Taylor is a Senior Analytical Chemist and Susana Da Silva Torres is a Post Doctoral Research Student in Pharmaceutical Sciences, Pfizer Worldwide R&D, Sandwich, UK.
- R. Hurd and R. Lane, “Principles of Very Low Power Electrochemical Control Devices”, J. Electrochem. Soc. 104 (12), 727–730 (1957).
- S. Weber and W. Purdy, “Electrochemical Detectors in Liquid Chromatography. A Short Review of Detector Design”, Ind. Eng. Chem. Prod. Res. Dev. 20 (4), 593–598 (1981).
- F. Lombardo and G. Campos,“How Do We Study Oxidative Chemical Stability In Discovery? Some Ideas, Trials, And Outcomes”, Biotechnology: Pharmaceutical Aspects, 1, 183–194 (2004).
- A.Baumann and U. Karst, “Online Electrochemistry/Mass Spectrometry in Drug Metabolism Studies: Principles and Applications”, Expert. Opin. Drug Metab. Toxicol. 6 (6) 715–731 (2010).
- S. Baertschi, P. Jansen and K. Alsante, “Pharmaceutical Stress Testing: Predicting Drug Degradation. (2nd Edition)”, Informa Health Care, New York, p. 10–48 (2011).
- T. Pluskal, T. Uehara and M. Yanagida, “Highly Accurate Chemical Formula Prediction Tool Utilizing High-Resolution Mass Spectra, MS/MS Fragmentation, Heuristic Rules, and Isotope Pattern Matching”, Anal. Chem. 84 (10), 4396–4403 (2012).
- D. Falck et al., “EC-SPE-Stripline-Nmr Analysis Of Reactive Products: A Feasibility Study”, Anal. Bioanal. Chem. 405(21), 6711–6720 (2013).
- B. Oshlack et al, “Stabilization of Naltrexone Compositions”, PCT Int. Appl. (2003), WO 2003077867 A2 20030925
- S. Da Silva Torres et al, “Rapid Synthesis Of Pharmaceutical Oxidation Products Using Electrochemistry: A Systematic Study Of N-Dealkylation Reactions Of Fesoterodine Using A Commercially Available Synthesis Cell“, Organic Process Research & Development (2014), in press

















