Eye on the Prize
The analytics required to prepare a Chemistry, Manufacturing, and Controls (CMC) file for a new bio-pharmaceutical takes expertise, organization, time and money. Here’s one company’s story of success.
Jean-Luc Jonniaux |
The story of Jetrea started back in 2000. At ThromboGenics, the proteolytic region of human plasmin was being investigated for its ability to dissolve blood clots during thrombosis (hence the company name), in collaboration with Desiré Collen at the Katholieke Universiteit Leuven, Belgium. A phase I clinical trial was initiated in 2002 using ocriplasmin, a genetically engineered version of the proteolytic region of human plasmin.
Then, physicians discovered that ocriplasmin can cleave the proteins that cause vitreous macular adhesion (VMA; see ‘Seeing is Believing’ sidebar). A phase II clinical trial was designed in 2004 to define the therapeutic window for the VMA treatment. When the decision was made to undertake a phase III clinical trial, ThromboGenics needed to scale up the manufacture of ocriplasmin drug substance (DS) and to invest in validation, in order to comply with good manufacturing practice (GMP). To achieve this, in 2007, manufacture was transferred to a new contract manufacturing organization (CMO) with larger capacity. The process was designed and characterised between 2007 and 2009, a period that included three production runs to validate commercial-scale production. This material was used for the phase III trial, which was completed by the end of 2009. The trial results were encouraging, and the decision was made to submit a biological license application (BLA) to the US Food and Drug Administration (FDA) and a marketing authorisation application (MAA) to the European Medicines Agency (EMA).The analytical data in these submission dossiers has a very strong bearing on the positive outcome of the process.
Analytics for Approval
Ocriplasmin is a small protein (27kDa) with several disulphide bridges. The challenge it presents is that it has autoproteolytic activity. Controlling the levels of product-related variants throughout the manufacturing process is essential.
Table 1 summarizes the properties that we measured, using validated methods, throughout the ocriplasmin production process. The critical raw material in recombinant protein production is the genetically manipulated cells themselves. Analytical requirements for these are well documented in the quality guidelines Q5 and Q6 from the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH; see www.ich.org/products/guidelines/). ICH is a useful organization that brings together the regulatory authorities and pharmaceutical industry of Europe, Japan and the US to discuss scientific and technical aspects of drug registration.
Briefly, we performed routine monitoring by in-process sample testing (Table 2). The production process consists of more than 10 steps and the submission dossier demonstrated the consistency of each step using validated purity and content methods. The absence of contaminants like endotoxin or host cell protein was also assessed by several validated methods. The quality attributes of the drug substance (DS) and drug product (DP) were verified with validated methods comparable to those for monoclonal antibodies, as laid out in the European Pharmacopoeia 5.2.
We also defined the stability of drug substance (DS) and drug product (DP) in the scaled-up product batches, demonstrated the photostabililty of these batches and compared them to the batches that had been generated for the clinical trials, which had been produced on a much smaller scale than those for commercial process. Additional characterization of ocriplasmin included solving the 1D, 2D and 3D structures, measuring the physico-chemical properties and assessing potency to hydrolyse physiological substrates.
| Studies | In Process | DS / DP | Ref. Standard |
|---|---|---|---|
| Process Design Space | (X) | (X) | |
| Characterisation | (X) | X | X |
| Release | X | X | X |
| Stability | X | X | |
| Photostability | X | ||
| Comparability clinical batch - commercial batch | X | X | |
| Extractable / leachable | X | ||
| Specifications | X | X | X |
| Process | Purity | Potency | Content | General properties | Identity | Contaminants + Impurities |
|---|---|---|---|---|---|---|
| Raw Materials | X | X | ||||
| Cell | X | X | X | X | X | |
| DS Process step | X | X | X | |||
| DS | X | X | X | X | X | X |
| DP Process step | X | X | ||||
| DP | X | X | X | X | X | X |
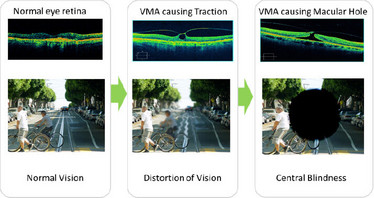
Seeing is Believing
Jetrea is a Non-Surgical Treatment of Vitreo-Macular Adhesion.
As people age, anatomical changes to the eye are common. One common ocular change is posterior vitreous detachment (PVD), in which the jelly-like part of the back of the eye, known as the vitreous, separates from the retina, which is a vital part of the eye that converts light into images by sending signals to the brain. Uniform detachments don’t disturb the vision but if the vitreous remains attached to a small area of the retina it can create a hole. When this happens in the retinal region specialized for sharp color vision, the macula, the vision of aged people is distorted with a decrease of visual acuity, leading possibly to blindness. This pathology is known as symptomatic vitreous macular adhesion (VMA).
Until recently, the only active treatment option available was surgery in which adhesions are dissected from the macular surface with aspiration of the vitreous. This ‘vitrectomy’ has frequent eye-associated complications as well as the risk of general anaesthesia in this age group. Post-vitrectomy, patients may also have to undergo a period of 4-6 weeks without being able to work, out of which 7-14 days may be in a ’head-down‘ position to enhance the success rate of the surgical procedure. Therefore, surgery is only used as an intervention when patients have, or are at risk of, severe visual disturbance and/or central blindness.
ThromboGenics has developed a first in class pharmacological treatment for symptomatic VMA. It consists of one injection of Jetrea into the eye.
Jetrea (ocriplasmin) is a truncated form of human plasmin. In the US, Jetrea is indicated for the treatment of symptomatic VMA. In Europe, Jetrea is indicated for the treatment of vitreomacular traction (VMT), including when associated with macular hole of diameter ≤ 400 microns.
Jetrea is a selective proteolytic enzyme that cleaves fibronectin, laminin and collagen, three major components of the vitreoretinal interface that play an important role in vitreomacular adhesion. It has been evaluated in two multi-center, randomized, double-masked Phase III trials conducted in the U.S. and Europe, involving 652 patients with vitreomacular adhesion. Both studies met the primary endpoint of resolution of VMA at day 28: 26.5 percent of patients treated with ocriplasmin saw resolution of VMA, compared with 10.1% of patients receiving placebo (p<0.01).
Jetrea is presented as a 2mL vial with 0.2mL solution stored at -20°C.
The low risk associated with a Jetrea injection compared to vitrectomy makes this treatment attractive for aged patients and does not limit surgery later if the vitreous macular detachment is not observed. The FDA has approved the product in October 2012 and EMA judged the benefit/risk balance to be positive in January 2013. Jetrea was launched in the US market in January 2013.
All existing and potential product-related variants had to be prepared and tested. These were either isolated from the process, or generated by accelerated degradation or de novo production using mutated genes. The variants were identified by mass spectroscopy (LC-MS) and their potency measured, in order to classify them as product-related substances or product-related impurities. In addition, the potential for ocriplasmin aggregation was investigated using several orthogonal methods. This is because recombinant protein aggregation is a known concern because of the immunological reactions they can induce, even though Jetrea is injected into the eye vitreous, where less immunological problems are anticipated in healthy individuals.
Such characterization data and the validated methods used to generate them are key components of the submission dossiers. They demonstrate an extended knowledge of process and properties pertaining to the molecule of interest.
ThromboGenics submitted the Jetrea dossiers in September 2011 to EMA and in April 2012 to FDA. Previous meetings with both agencies meant that we had aligned submission with the expectations of the agencies. However, as always when a drug is about to be commercialized, questions arose. The first set of EMA questions (called Day 120 Questions) were communicated in February 2012, and from the FDA in May 2012, and further sets of questions were received over the following four months. Concurrently, the FDA conducted pre-approval inspections at the CMOs contracted by ThromboGenics.
Finally, in October 2012, the FDA approved Jetrea for the US market. Some post-marketing commitments were made at this time. The very next day, EMA sent their second round of questions (Day 180 Questions), to which the company responded. The European Committee for Medicinal Products for Human Use (CHMP), which is responsible for preparing the Agency‘s opinions, then communicated a positive opinion on Jetrea. That was in January 2013; a decision from the European Commission is expected in March or April, 2013. In all, more than 100 CMC-related questions were handled during the assessment processes, most of them requiring additional analytical data.
Organizing for Success
We ascribe the success of the Jetrea submission to the positive clinical trial results, of course, but also to our strategy for fulfilling the expectations of FDA and EMA. To ensure maximal flexibility in responding to questions from the authorities and to provide validated methods in a timely manner, several QC and R&D laboratories were contracted to provide manufacturing and analytical services (see Figure 1). At any point in the process, work planned for one CMO could be transferred to another one to avoid delay.
This complex structure gave us the capability of delivering dossiers and responses in a timely fashion. Our rapid, coordinated approach was led by a small team that was solely dedicated to this purpose. The team members combined knowledge of, and experience in, regulatory, quality assurance, QC laboratory, GMP production, logistics and fundamental sciences. The group conducted short, efficient meetings with other parties that got right to the point. It had financial and operational freedom inside the company, and was allowed to take decisions and actions in less than a couple of hours. The ThromboGenics team was located in physical proximity to each other and had one common goal.
This type of business model is pivotal for a company the size of ThromboGenics. It is the only way to compile comprehensive BLA and MAA dossiers with the necessary complex, high quality information that is required to respond to the 100-plus CMC-related questions with speed and accuracy and, ultimately, to gain approval for a new therapy in the United States and Europe in parallel processes.
Jean-Luc Jonniaux worked for several years in the food industry. “Screening for microorganisms collected from all over the world to optimize production in 100m3 fermentors is fantastic training. You gain experience in genetics, physiology, process and analytics”, he says. Jean-Luc moved to the pharmaceutical world where he developed processes for virus, antibody and stem cell production for clinical trials. “The volumes processed are small but, like the bonsai tree, must be perfect,” he explains. “It requires all your attention and deep knowledge of the art. Analytic tools are essential to master the drug.” He put his combined experience to work in obtaining market authorization for a new biopharmaceutical.

















