Leveling Up LNP Analysis
Presenting a more convenient and environmentally friendly way to analyze the lipid content in lipid nanoparticles – a major challenge in the development of these promising drug delivery vehicles
| 4 min read
sponsored by Knauer
Lipid nanoparticles (LNPs) help deliver active pharmaceutical ingredients that cannot typically pass through the cell membrane – for example, mRNA – into cells. They were used for the rapid development and large-scale production of vaccines during the COVID-19 pandemic, which not only saved lives but also raised the profile of LNPs in the broader scientific community – even the general public. LNPs have gained interest as delivery systems for RNA as a therapeutic drug beyond vaccines – for treatment of cancer or of inherited disorders, for example. As new applications of LNPs are developed, quality control of the new therapeutics will have to evolve in tandem; however, not all methods needed for this task are currently covered by the pharmacopeias. In fact, some recent draft guidelines have suggested lengthy and solvent-consuming methods…
And that’s where KNAUER, as a producer of impingement jet mixing systems for the formulation of LNPs, comes in – providing the equipment and know-how for the quality control of LNP formulations with AZURA® HPLC systems. Here, we speak with Mareike Prüfer, Application Scientist at KNAUER, to find out about a recent study by KNAUER that demonstrates how the lipid composition of LNP formulations can be analyzed quickly and sustainably.
What are some of the analytical challenges associated with LNPs?
In research and in pharmaceutical production, LNPs are subjected to careful quality control. The identity, quality, and purity of the raw materials, especially the mRNA and lipids, as well as the quality and composition of the final product, must be verified. Many of the control criteria can be assayed using HPLC. However, the simultaneous analysis of lipids used in LNP formulations is challenging. They are highly hydrophobic and sometimes hardly elute from classical C18 columns. At the same time, some of the lipids are polydisperse, others are capable of different ionic interactions, and all those features can cause peak broadening. On top of that, the lipids lack chromophores and cannot be simply detected by a UV detector.
How does KNAUER approach these challenges?
For lipid detection, we decided to use an evaporative light scattering detector (ELSD), which offers simplicity in terms of operation and is also sensitive to quantify lipids and impurities in LNP formulations. With a clever choice of column, we were able to make the methods fast and convenient as well. In our newest application note, we show a rapid and sensitive isocratic method for the separation of lipids from LNPs.
Let’s talk about that application note on rapid analysis of lipid composition in LNP formulations… What inspired the study?
The USP published a method for assaying the lipid content in LNPs in their draft guidelines, but it is rather lengthy and consumes a high quantity of solvents, which are also modified with environmentally persistent TFA. We thought that there must be a more convenient and environmentally friendly way to analyze the lipid content – a method we could suggest to our customers and partners with good conscience.
What were your main findings?
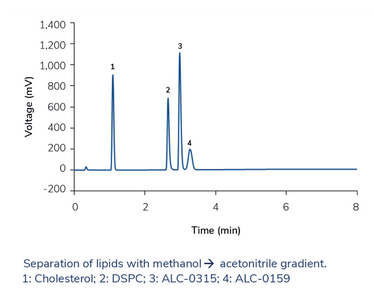
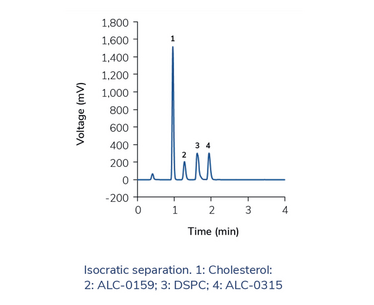
Instead of using C18 columns and long gradients with high elution strength, a less hydrophobic phase can be more effective, so we used phenyl-hexyl stationary phases. With the eluents methanol or acetonitrile alone, we did not reach complete elution of all lipids with a satisfactory peak shape. However, we found that when mixing both organic solvents, separation is much improved, and even better with a neutral modifier than with an organic acid. When optimizing the gradient, we noticed that, under certain conditions, most of the analytes were separated during the isocratic hold – and therefore, they could be separated in an isocratic run as well. We were able to optimize isocratic methods for lipid separation in only 2.5 min – without the need to re-equilibrate the column. The high and stable organic content of the mobile phase is also beneficial for ELSD detection because the evaporation temperature can be lowered, which increases sensitivity. We reached limits of quantification in the low nanogram range for all tested lipids.
Do you have any other "top tips" for LNP analysis?
Gradients are all around reverse-phase HPLC – but they are not always necessary, so it can be worth trying to reach separation with an isocratic method. And don’t forget to optimize your ELSD. The standard settings can be handy for method development, but sensitivity can be higher at a lower evaporation temperature.
What does the future of LNPs look like?
LNPs will become a new standard as a drug delivery system, and the efficient analysis of LNP components demand standardization as well. Researchers and the pharma industry will benefit from fast and simple methods that further speed up the development of new medicines. In the long run, it will be the patients who profit the most when LNPs come to their full potential and improve the treatment and prevention of many diseases.
Any final thoughts?
Stay open-minded during method development! An analytical scientist should always be ready to think outside of the box to discover a surprisingly easy solution for an analytical problem!
You can access the application note here

















